Factors secreted by human T lymphotropic virus type I (HTLV-I)-infected cells can enhance or inhibit replication of HIV-1 in HTLV-I-uninfected cells: implications for in vivo coinfection with HTLV-I and HIV-1 - PubMed (original) (raw)
Factors secreted by human T lymphotropic virus type I (HTLV-I)-infected cells can enhance or inhibit replication of HIV-1 in HTLV-I-uninfected cells: implications for in vivo coinfection with HTLV-I and HIV-1
H Moriuchi et al. J Exp Med. 1998.
Abstract
It remains controversial whether human T lymphotropic virus type I (HTLV-I) coinfection leads to more rapid progression of human immunodeficiency virus (HIV) disease in dually infected individuals. To investigate whether HTLV-I infection of certain cells can modulate HIV-1 infection of surrounding cells, primary CD4(+) T cells were treated with cell-free supernatants from HTLV-I-infected MT-2 cell cultures. The primary CD4+ T cells became resistant to macrophage (M)-tropic HIV-1 but highly susceptible to T cell (T)-tropic HIV-1. The CC chemokines RANTES (regulated on activation, normal T cell expressed and secreted), macrophage inflammatory protein (MIP)-1alpha, and MIP-1beta in the MT-2 cell supernatants were identified as the major suppressive factors for M-tropic HIV-1 as well as the enhancers of T-tropic HIV-1 infection, whereas soluble Tax protein increased susceptibility to both M- and T-tropic HIV-1. The effect of Tax or CC chemokines on T-tropic HIV-1 was mediated, at least in part, by increasing HIV Env-mediated fusogenicity. Our data suggest that the net effect of HTLV-I coinfection in HIV-infected individuals favors the transition from M- to T-tropic HIV phenotype, which is generally indicative of progressive HIV disease.
Figures
Figure 1
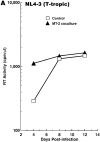
M-tropic HIV-1 infection is downregulated and T-tropic HIV-1 infection is enhanced in primary CD4+ T cells cocultured with HTLV-I–infected MT-2 cells in a transwell system. Primary CD4+ T cells isolated from HIV/HTLV seronegative individuals either were untreated or were cocultured with MT-2 cells in a transwell (0.2-μm pore membrane) culture for 3 d, and infected with either HIV-1NL4-3 (A) or HIV-1ADA (B). Cell-free supernatants were collected on days 4, 8, and 12 after infection and assayed for RT activity. Experiments were repeated twice with similar results.
Figure 1
M-tropic HIV-1 infection is downregulated and T-tropic HIV-1 infection is enhanced in primary CD4+ T cells cocultured with HTLV-I–infected MT-2 cells in a transwell system. Primary CD4+ T cells isolated from HIV/HTLV seronegative individuals either were untreated or were cocultured with MT-2 cells in a transwell (0.2-μm pore membrane) culture for 3 d, and infected with either HIV-1NL4-3 (A) or HIV-1ADA (B). Cell-free supernatants were collected on days 4, 8, and 12 after infection and assayed for RT activity. Experiments were repeated twice with similar results.
Figure 2
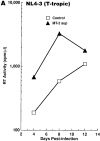
M-tropic HIV-1 infection is downregulated and T-tropic HIV-1 infection is enhanced over a 12-d culture period in primary CD4+ T cells treated continuously with MT-2 cell culture supernatants. Primary CD4+ T cells were pretreated with cell-free crude supernatants from either A3.01 cells (control) or MT-2 cells (MT-2 sup) at a 1: 1 ratio for 3 d before infection with HIV-1NL4-3 (A), HIV-189.6 (B), or HIV-1ADA (C). Culture medium was replaced with the same medium containing either control or MT-2 cell supernatant every 4 d after infection, and RT activity was measured. Representative results from three independent experiments are shown.
Figure 2
M-tropic HIV-1 infection is downregulated and T-tropic HIV-1 infection is enhanced over a 12-d culture period in primary CD4+ T cells treated continuously with MT-2 cell culture supernatants. Primary CD4+ T cells were pretreated with cell-free crude supernatants from either A3.01 cells (control) or MT-2 cells (MT-2 sup) at a 1: 1 ratio for 3 d before infection with HIV-1NL4-3 (A), HIV-189.6 (B), or HIV-1ADA (C). Culture medium was replaced with the same medium containing either control or MT-2 cell supernatant every 4 d after infection, and RT activity was measured. Representative results from three independent experiments are shown.
Figure 2
M-tropic HIV-1 infection is downregulated and T-tropic HIV-1 infection is enhanced over a 12-d culture period in primary CD4+ T cells treated continuously with MT-2 cell culture supernatants. Primary CD4+ T cells were pretreated with cell-free crude supernatants from either A3.01 cells (control) or MT-2 cells (MT-2 sup) at a 1: 1 ratio for 3 d before infection with HIV-1NL4-3 (A), HIV-189.6 (B), or HIV-1ADA (C). Culture medium was replaced with the same medium containing either control or MT-2 cell supernatant every 4 d after infection, and RT activity was measured. Representative results from three independent experiments are shown.
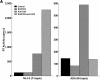
Figure 3
Soluble factors produced by MT-2 cells modulate early events in the HIV-1 replication cycle. Primary CD4+ T cells were pretreated with cell-free crude supernatants from either A3.01 cells (control) or MT-2 cells (MT-2 sup) at a 1:1 ratio for 3 d before infection with NL4-3-Luc-R−E− virus pseudotyped by Env from HIV-1HXB2, HIV-189.6, or HIV-1ADA. Luciferase activity in the infected cell lysates was measured on day 4 after infection. Representative results from three independent experiments are shown.
Figure 4
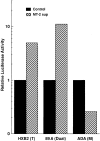
HTLV-I transactivator Tax protein and the CC chemokines RANTES, MIP-1α, and MIP-1β produced by MT-2 cells modulate HIV-1 infection. (A) MT-2 cells produce and secrete a soluble form of HTLV-I transactivator Tax protein. Crude supernatants from A3.01 cells (control; lane 1) or MT-2 cell supernatants treated with control serum (MT-2 sup; lane 2), mAbs to RANTES, MIP-1α, and MIP1β (−CC chemokines; lane 3), or anti-Tax antiserum (-Tax; lane 4) followed by protein A/G sepharose were immunoprecipitated with anti-Tax antiserum and protein A/G sepharose, subjected to 10% SDS-PAGE, and transferred to nitrocellulose membrane for Western blotting. The blot was probed with anti-Tax antiserum and horseradish peroxidase–conjugated protein A (Amersham Corp., Arlington Heights, IL). (B) MT-2 cells produce and secrete CC chemokines. Concentrations of RANTES, MIP-1α, and MIP-1β in the above supernatants were measured with ELISA. (C) The effect of subtraction of each component from MT-2 cell supernatants on HIV-1 infection. Primary CD4+ T cells were treated with the indicated supernatants at a 1:1 ratio for 3 d before infection with HIV-1NL4-3 or HIV-1ADA. Culture medium was replaced with the same medium every 4 d after infection, and RT activity was measured. Peak RT activities on day 12 are shown. Experiments were repeated three times with similar results.
Figure 4
HTLV-I transactivator Tax protein and the CC chemokines RANTES, MIP-1α, and MIP-1β produced by MT-2 cells modulate HIV-1 infection. (A) MT-2 cells produce and secrete a soluble form of HTLV-I transactivator Tax protein. Crude supernatants from A3.01 cells (control; lane 1) or MT-2 cell supernatants treated with control serum (MT-2 sup; lane 2), mAbs to RANTES, MIP-1α, and MIP1β (−CC chemokines; lane 3), or anti-Tax antiserum (-Tax; lane 4) followed by protein A/G sepharose were immunoprecipitated with anti-Tax antiserum and protein A/G sepharose, subjected to 10% SDS-PAGE, and transferred to nitrocellulose membrane for Western blotting. The blot was probed with anti-Tax antiserum and horseradish peroxidase–conjugated protein A (Amersham Corp., Arlington Heights, IL). (B) MT-2 cells produce and secrete CC chemokines. Concentrations of RANTES, MIP-1α, and MIP-1β in the above supernatants were measured with ELISA. (C) The effect of subtraction of each component from MT-2 cell supernatants on HIV-1 infection. Primary CD4+ T cells were treated with the indicated supernatants at a 1:1 ratio for 3 d before infection with HIV-1NL4-3 or HIV-1ADA. Culture medium was replaced with the same medium every 4 d after infection, and RT activity was measured. Peak RT activities on day 12 are shown. Experiments were repeated three times with similar results.
Figure 4
HTLV-I transactivator Tax protein and the CC chemokines RANTES, MIP-1α, and MIP-1β produced by MT-2 cells modulate HIV-1 infection. (A) MT-2 cells produce and secrete a soluble form of HTLV-I transactivator Tax protein. Crude supernatants from A3.01 cells (control; lane 1) or MT-2 cell supernatants treated with control serum (MT-2 sup; lane 2), mAbs to RANTES, MIP-1α, and MIP1β (−CC chemokines; lane 3), or anti-Tax antiserum (-Tax; lane 4) followed by protein A/G sepharose were immunoprecipitated with anti-Tax antiserum and protein A/G sepharose, subjected to 10% SDS-PAGE, and transferred to nitrocellulose membrane for Western blotting. The blot was probed with anti-Tax antiserum and horseradish peroxidase–conjugated protein A (Amersham Corp., Arlington Heights, IL). (B) MT-2 cells produce and secrete CC chemokines. Concentrations of RANTES, MIP-1α, and MIP-1β in the above supernatants were measured with ELISA. (C) The effect of subtraction of each component from MT-2 cell supernatants on HIV-1 infection. Primary CD4+ T cells were treated with the indicated supernatants at a 1:1 ratio for 3 d before infection with HIV-1NL4-3 or HIV-1ADA. Culture medium was replaced with the same medium every 4 d after infection, and RT activity was measured. Peak RT activities on day 12 are shown. Experiments were repeated three times with similar results.
Figure 5
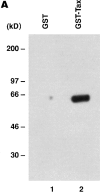
Purified Tax protein, in combination with anti-CD3 antibody, increases susceptibility to fusogenicity with Env from HIV-1. (A) GST–Tax fusion protein was purified as described in the Materials and Methods, subjected to 10% SDS-PAGE (lane 2), and transferred to nitrocellulose membrane for Western blotting, and then the blot was probed with anti-Tax antiserum and horseradish peroxidase–conjugated protein A. GST protein was prepared in the same manner and used as a control (lane 1). (B) GST–Tax fusion protein was taken up by the cells and transferred to the nucleus. Nuclear extracts were prepared from primary CD4+ T cells treated with either GST (100 ng/ml; lane 1) or GST–Tax (100 ng/ml) (lane 2), and analyzed in Western blotting as described above. The arrow indicates the position of GST–Tax protein. (C) Purified Tax protein in combination with anti-CD3 antibody enhances infectivity of virus pseudotyped by Envs from HIV-1HXB2, HIV-189.6, or HIV-1ADA. Primary CD4+ T cells were treated with either GST (20 ng/ ml) or GST–Tax protein (20 ng/ml) in the presence or absence of anti-CD3 mAb (OKT3 ascites 1:2000) for 3 d before infection. Representative results from four independent experiments are shown. (D) Purified Tax protein in combination with anti-CD3 antibody enhances HIV-1 Env-mediated cell-to-cell fusogenic activity. Primary CD4+ T cells were treated as above, infected with vTF7-3 (expressing T7 polymerase), and mixed with BSC-1 cells infected with vCB21R (encoding lacZ gene under the control of the T7 promoter) as well as rVV expressing the indicated HIV-1 Env. Fusion index indicates OD570 value obtained for HIV-1 Env-mediated cell fusion relative to the background value obtained for nonfusogenic uncleaved Env-mediated cell fusion. Representative results from three independent experiments are shown.
Figure 5
Purified Tax protein, in combination with anti-CD3 antibody, increases susceptibility to fusogenicity with Env from HIV-1. (A) GST–Tax fusion protein was purified as described in the Materials and Methods, subjected to 10% SDS-PAGE (lane 2), and transferred to nitrocellulose membrane for Western blotting, and then the blot was probed with anti-Tax antiserum and horseradish peroxidase–conjugated protein A. GST protein was prepared in the same manner and used as a control (lane 1). (B) GST–Tax fusion protein was taken up by the cells and transferred to the nucleus. Nuclear extracts were prepared from primary CD4+ T cells treated with either GST (100 ng/ml; lane 1) or GST–Tax (100 ng/ml) (lane 2), and analyzed in Western blotting as described above. The arrow indicates the position of GST–Tax protein. (C) Purified Tax protein in combination with anti-CD3 antibody enhances infectivity of virus pseudotyped by Envs from HIV-1HXB2, HIV-189.6, or HIV-1ADA. Primary CD4+ T cells were treated with either GST (20 ng/ ml) or GST–Tax protein (20 ng/ml) in the presence or absence of anti-CD3 mAb (OKT3 ascites 1:2000) for 3 d before infection. Representative results from four independent experiments are shown. (D) Purified Tax protein in combination with anti-CD3 antibody enhances HIV-1 Env-mediated cell-to-cell fusogenic activity. Primary CD4+ T cells were treated as above, infected with vTF7-3 (expressing T7 polymerase), and mixed with BSC-1 cells infected with vCB21R (encoding lacZ gene under the control of the T7 promoter) as well as rVV expressing the indicated HIV-1 Env. Fusion index indicates OD570 value obtained for HIV-1 Env-mediated cell fusion relative to the background value obtained for nonfusogenic uncleaved Env-mediated cell fusion. Representative results from three independent experiments are shown.
Figure 5
Purified Tax protein, in combination with anti-CD3 antibody, increases susceptibility to fusogenicity with Env from HIV-1. (A) GST–Tax fusion protein was purified as described in the Materials and Methods, subjected to 10% SDS-PAGE (lane 2), and transferred to nitrocellulose membrane for Western blotting, and then the blot was probed with anti-Tax antiserum and horseradish peroxidase–conjugated protein A. GST protein was prepared in the same manner and used as a control (lane 1). (B) GST–Tax fusion protein was taken up by the cells and transferred to the nucleus. Nuclear extracts were prepared from primary CD4+ T cells treated with either GST (100 ng/ml; lane 1) or GST–Tax (100 ng/ml) (lane 2), and analyzed in Western blotting as described above. The arrow indicates the position of GST–Tax protein. (C) Purified Tax protein in combination with anti-CD3 antibody enhances infectivity of virus pseudotyped by Envs from HIV-1HXB2, HIV-189.6, or HIV-1ADA. Primary CD4+ T cells were treated with either GST (20 ng/ ml) or GST–Tax protein (20 ng/ml) in the presence or absence of anti-CD3 mAb (OKT3 ascites 1:2000) for 3 d before infection. Representative results from four independent experiments are shown. (D) Purified Tax protein in combination with anti-CD3 antibody enhances HIV-1 Env-mediated cell-to-cell fusogenic activity. Primary CD4+ T cells were treated as above, infected with vTF7-3 (expressing T7 polymerase), and mixed with BSC-1 cells infected with vCB21R (encoding lacZ gene under the control of the T7 promoter) as well as rVV expressing the indicated HIV-1 Env. Fusion index indicates OD570 value obtained for HIV-1 Env-mediated cell fusion relative to the background value obtained for nonfusogenic uncleaved Env-mediated cell fusion. Representative results from three independent experiments are shown.
Figure 5
Purified Tax protein, in combination with anti-CD3 antibody, increases susceptibility to fusogenicity with Env from HIV-1. (A) GST–Tax fusion protein was purified as described in the Materials and Methods, subjected to 10% SDS-PAGE (lane 2), and transferred to nitrocellulose membrane for Western blotting, and then the blot was probed with anti-Tax antiserum and horseradish peroxidase–conjugated protein A. GST protein was prepared in the same manner and used as a control (lane 1). (B) GST–Tax fusion protein was taken up by the cells and transferred to the nucleus. Nuclear extracts were prepared from primary CD4+ T cells treated with either GST (100 ng/ml; lane 1) or GST–Tax (100 ng/ml) (lane 2), and analyzed in Western blotting as described above. The arrow indicates the position of GST–Tax protein. (C) Purified Tax protein in combination with anti-CD3 antibody enhances infectivity of virus pseudotyped by Envs from HIV-1HXB2, HIV-189.6, or HIV-1ADA. Primary CD4+ T cells were treated with either GST (20 ng/ ml) or GST–Tax protein (20 ng/ml) in the presence or absence of anti-CD3 mAb (OKT3 ascites 1:2000) for 3 d before infection. Representative results from four independent experiments are shown. (D) Purified Tax protein in combination with anti-CD3 antibody enhances HIV-1 Env-mediated cell-to-cell fusogenic activity. Primary CD4+ T cells were treated as above, infected with vTF7-3 (expressing T7 polymerase), and mixed with BSC-1 cells infected with vCB21R (encoding lacZ gene under the control of the T7 promoter) as well as rVV expressing the indicated HIV-1 Env. Fusion index indicates OD570 value obtained for HIV-1 Env-mediated cell fusion relative to the background value obtained for nonfusogenic uncleaved Env-mediated cell fusion. Representative results from three independent experiments are shown.
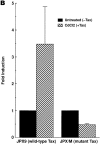
Figure 6
Tax protein expressed in a CD4+ Jurkat T cell line enhances HIV-1 Env-mediated cell-to-cell fusion efficiency. (A) tax is inducibly expressed in JPX9 cells (Jurkat cells stably transfected with wild-type HTLV-I Tax protein under the control of the metallothionein promoter) and JPX/M cells (Jurkat cells stably transfected with a nonactive mutant form of Tax). JPX9 and JPX/M cells were treated with CdCl2 (10 μM), and total RNA was prepared from cells before treatment (day 0) or on day 1 or 2 after treatment. Total RNA from MT-2 cells was also prepared as a positive control. 15 μg of total RNA was analyzed in Northern blotting using a probe specific to the tax gene (top). Ethidium bromide staining of the gel is presented (bottom), showing the comparable levels of 18S rRNA loaded. (B) Fusogenicity of JPX9 cells increases after induction of Tax expression. JPX9 and JPX/M cells were either untreated or treated with CdCl2 for 2 d, and cell–cell fusion assays were performed for the measurement of HIV-1 IIIB Env-mediated fusogenic activity. Fold induction indicates fusogenic activity mediated by Tax (+Tax) relative to the baseline (−Tax) fusogenic activity of each cell line. Results are means ± SD of four independent experiments.
Figure 6
Tax protein expressed in a CD4+ Jurkat T cell line enhances HIV-1 Env-mediated cell-to-cell fusion efficiency. (A) tax is inducibly expressed in JPX9 cells (Jurkat cells stably transfected with wild-type HTLV-I Tax protein under the control of the metallothionein promoter) and JPX/M cells (Jurkat cells stably transfected with a nonactive mutant form of Tax). JPX9 and JPX/M cells were treated with CdCl2 (10 μM), and total RNA was prepared from cells before treatment (day 0) or on day 1 or 2 after treatment. Total RNA from MT-2 cells was also prepared as a positive control. 15 μg of total RNA was analyzed in Northern blotting using a probe specific to the tax gene (top). Ethidium bromide staining of the gel is presented (bottom), showing the comparable levels of 18S rRNA loaded. (B) Fusogenicity of JPX9 cells increases after induction of Tax expression. JPX9 and JPX/M cells were either untreated or treated with CdCl2 for 2 d, and cell–cell fusion assays were performed for the measurement of HIV-1 IIIB Env-mediated fusogenic activity. Fold induction indicates fusogenic activity mediated by Tax (+Tax) relative to the baseline (−Tax) fusogenic activity of each cell line. Results are means ± SD of four independent experiments.
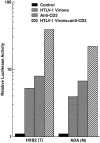
Figure 7
Recombinant CC chemokines, in combination with anti-CD3 antibody, enhance replication of and fusogenicity with Envs from T-tropic HIV-1. (A) Primary CD4+ T cells were either untreated or treated with CC chemokines (200 ng/ml RANTES [R&D Systems]), anti-CD3 antibody, or both for 3 d before infection with HIV-1NL4-3 or HIV-1ADA. Representative results from three independent experiments are shown. (B) Primary CD4+ T cells were treated as in A, and infected with vTF7-3 (fusion targets), and CD4+ T cells stimulated with anti-CD3 were infected with vCB21R as well as rVV expressing HIV-1NL4-3 Env (fusion effectors). Results were means ± SD from three independent experiments.
Figure 7
Recombinant CC chemokines, in combination with anti-CD3 antibody, enhance replication of and fusogenicity with Envs from T-tropic HIV-1. (A) Primary CD4+ T cells were either untreated or treated with CC chemokines (200 ng/ml RANTES [R&D Systems]), anti-CD3 antibody, or both for 3 d before infection with HIV-1NL4-3 or HIV-1ADA. Representative results from three independent experiments are shown. (B) Primary CD4+ T cells were treated as in A, and infected with vTF7-3 (fusion targets), and CD4+ T cells stimulated with anti-CD3 were infected with vCB21R as well as rVV expressing HIV-1NL4-3 Env (fusion effectors). Results were means ± SD from three independent experiments.
Figure 8
HTLV-I virions, in combination with anti-CD3 antibody, enhance replication of HIV-1. Primary CD4+ T cells were either untreated or treated with HTLV-I particles (1 μg/ml protein), anti-CD3 antibody, or both for 3 d before infection with NL4-3-Luc-R−E− virus pseudotyped by Env from HIV-1HXB2 or HIV-1ADA. Similar results were obtained twice.
Similar articles
- Inhibition of HIV type 1 replication by human T lymphotropic virus types 1 and 2 Tax proteins in vitro.
Barrios CS, Castillo L, Giam CZ, Wu L, Beilke MA. Barrios CS, et al. AIDS Res Hum Retroviruses. 2013 Jul;29(7):1061-7. doi: 10.1089/aid.2013.0027. Epub 2013 Mar 29. AIDS Res Hum Retroviruses. 2013. PMID: 23464580 Free PMC article. - Pseudotyping of HIV-1 with Human T-Lymphotropic Virus 1 (HTLV-1) Envelope Glycoprotein during HIV-1-HTLV-1 Coinfection Facilitates Direct HIV-1 Infection of Female Genital Epithelial Cells: Implications for Sexual Transmission of HIV-1.
Tang Y, George AM, Petrechko O, Nouvet FJ, Sweet SD, Tanaka Y, Imbiakha BS, Jiang G, Gao W, Anastos K, Hildreth JEK. Tang Y, et al. mSphere. 2018 Apr 4;3(2):e00038-18. doi: 10.1128/mSphere.00038-18. Print 2018 Apr 25. mSphere. 2018. PMID: 29624497 Free PMC article. - Molecular and cellular interactions of HIV-1/HTLV coinfection and impact on AIDS progression.
Casoli C, Pilotti E, Bertazzoni U. Casoli C, et al. AIDS Rev. 2007 Jul-Sep;9(3):140-9. AIDS Rev. 2007. PMID: 17982939 Review. - HIV/human T-cell lymphotropic virus coinfection revisited: impact on AIDS progression.
Brites C, Sampalo J, Oliveira A. Brites C, et al. AIDS Rev. 2009 Jan-Mar;11(1):8-16. AIDS Rev. 2009. PMID: 19290030 Review.
Cited by
- Inhibition of HIV type 1 replication by human T lymphotropic virus types 1 and 2 Tax proteins in vitro.
Barrios CS, Castillo L, Giam CZ, Wu L, Beilke MA. Barrios CS, et al. AIDS Res Hum Retroviruses. 2013 Jul;29(7):1061-7. doi: 10.1089/aid.2013.0027. Epub 2013 Mar 29. AIDS Res Hum Retroviruses. 2013. PMID: 23464580 Free PMC article. - Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion.
Gordon CJ, Muesing MA, Proudfoot AE, Power CA, Moore JP, Trkola A. Gordon CJ, et al. J Virol. 1999 Jan;73(1):684-94. doi: 10.1128/JVI.73.1.684-694.1999. J Virol. 1999. PMID: 9847374 Free PMC article. - The Role of Chemokines in the Pathogenesis of HTLV-1.
Zargari R, Mahdifar M, Mohammadi A, Vahidi Z, Hassanshahi G, Rafatpanah H. Zargari R, et al. Front Microbiol. 2020 Mar 13;11:421. doi: 10.3389/fmicb.2020.00421. eCollection 2020. Front Microbiol. 2020. PMID: 32231656 Free PMC article. Review. - Exposure to bacterial products renders macrophages highly susceptible to T-tropic HIV-1.
Moriuchi M, Moriuchi H, Turner W, Fauci AS. Moriuchi M, et al. J Clin Invest. 1998 Oct 15;102(8):1540-50. doi: 10.1172/JCI4151. J Clin Invest. 1998. PMID: 9788967 Free PMC article. - The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity.
Trkola A, Gordon C, Matthews J, Maxwell E, Ketas T, Czaplewski L, Proudfoot AE, Moore JP. Trkola A, et al. J Virol. 1999 Aug;73(8):6370-9. doi: 10.1128/JVI.73.8.6370-6379.1999. J Virol. 1999. PMID: 10400729 Free PMC article.
References
- Zhu T, Mo H, Wang N, Nam DS, Cao Y, Koup RA, Ho DD. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials