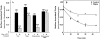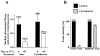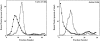Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains - PubMed (original) (raw)
Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains
P A Orlandi et al. J Cell Biol. 1998.
Abstract
The mechanism by which cholera toxin (CT) is internalized from the plasma membrane before its intracellular reduction and subsequent activation of adenylyl cyclase is not well understood. Ganglioside GM1, the receptor for CT, is predominantly clustered in detergent-insoluble glycolipid rafts and in caveolae, noncoated, cholesterol-rich invaginations on the plasma membrane. In this study, we used filipin, a sterol-binding agent that disrupts caveolae and caveolae-like structures, to explore their role in the internalization and activation of CT in CaCo-2 human intestinal epithelial cells. When toxin internalization was quantified, only 33% of surface-bound toxin was internalized by filipin-treated cells within 1 h compared with 79% in untreated cells. However, CT activation as determined by its reduction to form the A1 peptide and CT activity as measured by cyclic AMP accumulation were inhibited in filipin-treated cells. Another sterol-binding agent, 2-hydroxy-beta-cyclodextrin, gave comparable results. The cationic amphiphilic drug chlorpromazine, an inhibitor of clathrin-dependent, receptor-mediated endocytosis, however, affected neither CT internalization, activation, nor activity in contrast to its inhibitory effects on diphtheria toxin cytotoxicity. As filipin did not inhibit the latter, the two drugs appeared to distinguish between caveolae- and coated pit-mediated processes. In addition to its effects in CaCo-2 cells that express low levels of caveolin, filipin also inhibited CT activity in human epidermoid carcinoma A431 and Jurkat T lymphoma cells that are, respectively, rich in or lack caveolin. Thus, filipin inhibition correlated more closely with alterations in the biochemical characteristics of CT-bound membranes due to the interactions of filipin with cholesterol rather than with the expressed levels of caveolin and caveolar structure. Our results indicated that the internalization and activation of CT was dependent on and mediated through cholesterol- and glycolipid-rich microdomains at the plasma membrane rather than through a specific morphological structure and that these glycolipid microdomains have the necessary components required to mediate endocytosis.
Figures
Figure 1
Effect of filipin and chlorpromazine on the internalization of surface-bound CT in CaCo-2 cells. (A) Cells were incubated for 1 h at 37°C in MEM, Hepes, and 0.01% BSA with no addition; 1 μg/ml filipin; 10 μg/ml chlorpromazine; or both. Then the cells were chilled to 4°C and incubated with 10 nM CT for 1 h in the same medium. Cells were either washed and exposed to anti-CT-A1 antibodies for 1 h at 4°C or incubated in medium with the same additions for 1 h at 37°C before antibody exposure. Immunodetection of surface-bound CT was quantified with 125I-protein A as described in Materials and Methods. (B) Time course of CT internalization in untreated (•) and filipin-treated (○) cells.
Figure 2
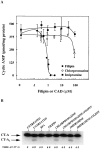
Inhibition of toxin activity and activation in CaCo-2 cells by filipin but not CADs. (A) CaCo-2 cells were treated with increasing concentrations of filipin (•), chlorpromazine (○), or imipramine (▪) for 1 h at 37°C. The cells were then incubated an additional 2 h at 37°C with 0.03 nM CT and assayed for cAMP accumulation as described in Materials and Methods. (B) CaCo-2 cells were incubated for 1 h at 37°C in the absence (UNTREATED) or presence of the indicated effector (1 μg/ml BFA and filipin; 10 μg/ml chlorpromazine and imipramine). The cells then were incubated with 125I-CT for 1 h at 4°C in media containing the effector, washed and either harvested or incubated an additional 1 h at 37°C in fresh medium with effectors. All cells were then analyzed for the formation of CT-A1 peptide as described in Materials and Methods.
Figure 3
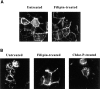
Effects of filipin and chlorpromazine on the distribution of rhodamine-conjugated CT-B in CaCo-2 cells detected by direct fluorescence microscopy. Cells were incubated for 1 h at 37°C in MEM, Hepes, and 0.01% BSA with no addition; 1 μg/ml filipin; or 25 μg/ml chlorpromazine. The cells were then labeled with 5 nM Rh-CT-B for 30 min at 15°C in fresh medium in the presence or absence of the same effectors. Cells were washed once and incubated an additional 30 min at 4°C (A) or 37°C (B) in fresh medium with effectors and fixed_._ The distribution of Rh-CT-B was then visualized by direct fluorescence as described in Materials and Methods.
Figure 4
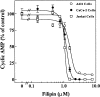
Comparison of the concentration-dependent effects of filipin on CT activity in CaCo-2 (•), A431 (○) and Jurkat (□) cells. Cells were treated with increasing concentrations of filipin for 1 h at 37°C, then incubated an additional 2 h at 37°C with 0.03 nM CT and assayed for cAMP accumulation as described in Materials and Methods.
Figure 5
Selective effects of filipin and chlorpromazine on the activity of cholera and diphtheria toxins. CaCo-2 and A431 cells were incubated in the absence or presence of 1 μg/ml filipin or 25 μg/ml chlorpromazine for 1 h at 37°C. Then the cells were exposed to either 0.03 nM CT or 100 ng/ml DT for an additional 2 h at 37°C and assayed for CT stimulation of cAMP accumulation or DT inhibition of protein synthesis as described in Materials and Methods. The results represent the mean ± SEM of two experiments, each done in triplicate. Absolute inhibition of protein synthesis in DT-treated cells was between 30–50%.
Figure 6
Effects of cyclodextrin on CT internalization and activation. CaCo-2 cells were cultured for 48 h in serum-free medium containing 0.1% BSA and then incubated for 4 h in serum-free medium containing 0.01% BSA without and with 100 mg/ml cyclodextrin at 37°C. Cells were then assayed for either CT internalization (A) as described in the legend to Fig. 1; or CT and DT cytotoxicities (B) as described in the legend to Fig. 5. Absolute inhibition of protein synthesis in DT-treated cells was 45%.
Figure 7
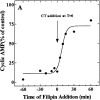
Filipin-mediated inhibition of CT activity in CaCo-2 cells is both rapid (A) and reversible (B and C). (A) Filipin (1 μg/ml) was added to cells either before (− values) or after (+ values) the addition of CT (0.03 nM, final). All the cells were exposed to the toxin for 2 h at 37°C and assayed for the accumulation of cAMP. Results are expressed as a percentage of cAMP formed in the absence of filipin. (B) Cells were treated in the absence (filled bars) or presence (open and _cross_-hatched bars) of 1 μg/ml filipin for the indicated times at 37°C, chilled to 4°C, and incubated with 10 nM CT for 1 h ± filipin. Cells were either exposed to anti-CT-A1 at 4°C (zero time) or incubated at 37°C with the same additions for the indicated times before antibody exposure. Some of the filipin-treated cells (hatched bars) were washed and incubated in filipin-free medium for an additional 60 and 120 min at 37°C before the addition of antibody at 4°C. Immunodetection of surface-bound CT was quantified with 125I-protein A as described in Materials and Methods. (C) Cells were treated in the presence (♦, ○, □) or absence (•) of 1 μg/ml filipin for 1 h at 37°C and some of the cells were either washed briefly (○); or, for 30 min (□) in filipin-free medium at 37°C. Then all of the cells were incubated with 0.03 nM CT for the indicated times at 37°C and assayed for cAMP accumulation as described in Materials and Methods.
Figure 7
Filipin-mediated inhibition of CT activity in CaCo-2 cells is both rapid (A) and reversible (B and C). (A) Filipin (1 μg/ml) was added to cells either before (− values) or after (+ values) the addition of CT (0.03 nM, final). All the cells were exposed to the toxin for 2 h at 37°C and assayed for the accumulation of cAMP. Results are expressed as a percentage of cAMP formed in the absence of filipin. (B) Cells were treated in the absence (filled bars) or presence (open and _cross_-hatched bars) of 1 μg/ml filipin for the indicated times at 37°C, chilled to 4°C, and incubated with 10 nM CT for 1 h ± filipin. Cells were either exposed to anti-CT-A1 at 4°C (zero time) or incubated at 37°C with the same additions for the indicated times before antibody exposure. Some of the filipin-treated cells (hatched bars) were washed and incubated in filipin-free medium for an additional 60 and 120 min at 37°C before the addition of antibody at 4°C. Immunodetection of surface-bound CT was quantified with 125I-protein A as described in Materials and Methods. (C) Cells were treated in the presence (♦, ○, □) or absence (•) of 1 μg/ml filipin for 1 h at 37°C and some of the cells were either washed briefly (○); or, for 30 min (□) in filipin-free medium at 37°C. Then all of the cells were incubated with 0.03 nM CT for the indicated times at 37°C and assayed for cAMP accumulation as described in Materials and Methods.
Figure 7
Filipin-mediated inhibition of CT activity in CaCo-2 cells is both rapid (A) and reversible (B and C). (A) Filipin (1 μg/ml) was added to cells either before (− values) or after (+ values) the addition of CT (0.03 nM, final). All the cells were exposed to the toxin for 2 h at 37°C and assayed for the accumulation of cAMP. Results are expressed as a percentage of cAMP formed in the absence of filipin. (B) Cells were treated in the absence (filled bars) or presence (open and _cross_-hatched bars) of 1 μg/ml filipin for the indicated times at 37°C, chilled to 4°C, and incubated with 10 nM CT for 1 h ± filipin. Cells were either exposed to anti-CT-A1 at 4°C (zero time) or incubated at 37°C with the same additions for the indicated times before antibody exposure. Some of the filipin-treated cells (hatched bars) were washed and incubated in filipin-free medium for an additional 60 and 120 min at 37°C before the addition of antibody at 4°C. Immunodetection of surface-bound CT was quantified with 125I-protein A as described in Materials and Methods. (C) Cells were treated in the presence (♦, ○, □) or absence (•) of 1 μg/ml filipin for 1 h at 37°C and some of the cells were either washed briefly (○); or, for 30 min (□) in filipin-free medium at 37°C. Then all of the cells were incubated with 0.03 nM CT for the indicated times at 37°C and assayed for cAMP accumulation as described in Materials and Methods.
Figure 8
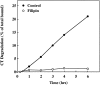
Filipin inhibits degradation of CT by CaCo-2 cells. Untreated cells (•) and cells treated with 1 μg/ml filipin (○) were incubated with 125I-CT at 4°C, washed to remove any unbound toxin, and incubated with fresh medium with or without filipin at 37°C for the indicated times. Toxin degradation was determined by the levels of TCA-soluble radioactivity that accumulated in the culture medium with time.
Figure 9
Analysis of Triton X-100 extracts from CaCo-2 and Jurkat cells on sucrose gradients. Untreated (•) and 1 μg/ml filipin-treated (○) CaCo-2 and Jurkat cells were incubated with 125I-CT for 1 h at 4°C, washed in PBS, scraped, and pelleted. After the cells were extracted for 30 min at 4°C in 1% Triton X-100 solution ± 1 μg/ml filipin, the extracts were centrifuged on a 10–30% linear sucrose gradient as described in Materials and Methods. Fractions (∼0.5 ml) were collected from the top of the gradient and counted for 125I.
Similar articles
- Brefeldin A blocks the response of cultured cells to cholera toxin. Implications for intracellular trafficking in toxin action.
Orlandi PA, Curran PK, Fishman PH. Orlandi PA, et al. J Biol Chem. 1993 Jun 5;268(16):12010-6. J Biol Chem. 1993. PMID: 8389369 - Ganglioside structure dictates signal transduction by cholera toxin and association with caveolae-like membrane domains in polarized epithelia.
Wolf AA, Jobling MG, Wimer-Mackin S, Ferguson-Maltzman M, Madara JL, Holmes RK, Lencer WI. Wolf AA, et al. J Cell Biol. 1998 May 18;141(4):917-27. doi: 10.1083/jcb.141.4.917. J Cell Biol. 1998. PMID: 9585411 Free PMC article. - Internalization of cholera toxin by different endocytic mechanisms.
Torgersen ML, Skretting G, van Deurs B, Sandvig K. Torgersen ML, et al. J Cell Sci. 2001 Oct;114(Pt 20):3737-47. doi: 10.1242/jcs.114.20.3737. J Cell Sci. 2001. PMID: 11707525 - Floating cholera toxin into epithelial cells: functional association with caveolae-like detergent-insoluble membrane microdomains.
Badizadegan K, Wolf AA, Rodighiero C, Jobling M, Hirst TR, Holmes RK, Lencer WI. Badizadegan K, et al. Int J Med Microbiol. 2000 Oct;290(4-5):403-8. doi: 10.1016/S1438-4221(00)80052-1. Int J Med Microbiol. 2000. PMID: 11111918 Review. - Caveolae--an alternative endocytotic pathway for targeted drug delivery.
Bathori G, Cervenak L, Karadi I. Bathori G, et al. Crit Rev Ther Drug Carrier Syst. 2004;21(2):67-95. doi: 10.1615/critrevtherdrugcarriersyst.v21.i2.10. Crit Rev Ther Drug Carrier Syst. 2004. PMID: 15202927 Review.
Cited by
- Vaccine delivery by penetratin: mechanism of antigen presentation by dendritic cells.
Pouniotis D, Tang CK, Apostolopoulos V, Pietersz G. Pouniotis D, et al. Immunol Res. 2016 Aug;64(4):887-900. doi: 10.1007/s12026-016-8799-5. Immunol Res. 2016. PMID: 27138940 - Lipid-based liquid crystalline nanoparticles as oral drug delivery vehicles for poorly water-soluble drugs: cellular interaction and in vivo absorption.
Zeng N, Gao X, Hu Q, Song Q, Xia H, Liu Z, Gu G, Jiang M, Pang Z, Chen H, Chen J, Fang L. Zeng N, et al. Int J Nanomedicine. 2012;7:3703-18. doi: 10.2147/IJN.S32599. Epub 2012 Jul 13. Int J Nanomedicine. 2012. PMID: 22888230 Free PMC article. - Increased PIP3 activity blocks nanoparticle mRNA delivery.
Paunovska K, Da Silva Sanchez A, Foster MT, Loughrey D, Blanchard EL, Islam FZ, Gan Z, Mantalaris A, Santangelo PJ, Dahlman JE. Paunovska K, et al. Sci Adv. 2020 Jul 22;6(30):eaba5672. doi: 10.1126/sciadv.aba5672. eCollection 2020 Jul. Sci Adv. 2020. PMID: 32743074 Free PMC article. - Analysis of CD44-containing lipid rafts: Recruitment of annexin II and stabilization by the actin cytoskeleton.
Oliferenko S, Paiha K, Harder T, Gerke V, Schwärzler C, Schwarz H, Beug H, Günthert U, Huber LA. Oliferenko S, et al. J Cell Biol. 1999 Aug 23;146(4):843-54. doi: 10.1083/jcb.146.4.843. J Cell Biol. 1999. PMID: 10459018 Free PMC article. - Mycobacterium tuberculosis Primary Infection and Dissemination: A Critical Role for Alveolar Epithelial Cells.
Ryndak MB, Laal S. Ryndak MB, et al. Front Cell Infect Microbiol. 2019 Aug 21;9:299. doi: 10.3389/fcimb.2019.00299. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31497538 Free PMC article. Review.
References
- Anderson RGW, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. - PubMed
- Beaumelle B, Bensammar L, Bienvenüe A. Selective translocation of the A chain of diphtheria toxin across the membrane of purified endosomes. J Biol Chem. 1992;267:11525–11531. - PubMed
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical surface. Cell. 1992;68:533–544. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials