MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes - PubMed (original) (raw)
MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes
T H Vu et al. Cell. 1998.
Abstract
Homozygous mice with a null mutation in the MMP-9/gelatinase B gene exhibit an abnormal pattern of skeletal growth plate vascularization and ossification. Although hypertrophic chondrocytes develop normally, apoptosis, vascularization, and ossification are delayed, resulting in progressive lengthening of the growth plate to about eight times normal. After 3 weeks postnatal, aberrant apoptosis, vascularization, and ossification compensate to remodel the enlarged growth plate and ultimately produce an axial skeleton of normal appearance. Transplantation of wild-type bone marrow cells rescues vascularization and ossification in gelatinase B-null growth plates, indicating that these processes are mediated by gelatinase B-expressing cells of bone marrow origin, designated chondroclasts. Growth plates from gelatinase B-null mice in culture show a delayed release of an angiogenic activator, establishing a role for this proteinase in controlling angiogenesis.
Figures
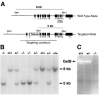
Figure 1. Generation of Gelatinase B–Null Mice
(A) Genomic organization of the wild-type and targeted alleles after homologous recombination. The location of the fragment used as a probe in Southern blotting is shown, as well as the sizes of the two XbaI fragments detected for wild-type and targeted alleles. (B) Southern blot analysis of genomic DNA. Fragments corresponding to wild-type (8 kb) and targeted (5 kb) alleles are shown. (C) Gelatin zymographic analysis of speen cell lysates. Gelatinolytic activity corresponding to gelatinase B is shown.
Figure 2. Histological Analysis of the Metatarsals of Wild-Type and Gelatinase B–Null Mice
(A) Diagram of the growth plate showing different cartilage zones. (B) Histological sections of 2-week-old metatarsals stained with hematoxylin/fast green/safranin O. (ep) epiphysis; (c) reserve, proliferation, and maturation zones; (hc) hypertrophic cartilage; (tb) trabecular bone; (bm) bone marrow. Scale bar = 200 μm. (C) Histological sections of 3-week-old metatarsals. Magnified view on the right lower panel shows cortical bone surrounding the hypertrophic cartilage in gelatinase B–null mice (outlined by white arrows). Black arrows point to capillaries invading from the cortical bone. (hc) hypertrophic cartilage; (tb) trabecular bone. (D) Histological sections of 4-week-old metatarsals showing aberrant ossification process in the gelatinase B–null mice (arrows in upper right panel). The lower left panel shows a bone whose hypertrophic cartilage has been almost completely ossified giving the appearance of osteopetrosis (arrows). Magnified view on the lower right panel shows a vessel invading from the side (arrow). (E) Von Kossa staining of 2-week-old metatarsal growth plates. Calcium deposits stain black. (F) Histological sections of 8-week-old metatarsals.
Figure 3. BrdU Labeling and In Situ Hybridization with Chondrocyte Markers
Left column panels show metatarsals from 2-week-old wild type and right column panels show those from gelatinase B–null mice. Cells that have incorporated BrdU stain brown. Arrow points to the secondary site of osssification. Zones of expression of Indian hedgehog (ihh), bone morphogenetic protein-6 (bmp-6), collagen type II (col2A1), and collagen type X (colXA1) are shown by dark-field photography of in situ hybridization with antisense mRNA probes. (hc) hypertrophic cartilage. Scale bar = 200 μm.
Figure 4. Apoptotic Cells in the Metatarsal Growth Plate
(A and B) Propidium iodide (PI) and ApopTag (Apop) staining of 1- and 2-week-old metatarsal growth plates from wild-type and gelatinase B–null mice. Arrows point to terminal hypertrophic chondrocytes that are apoptotic. Apoptotic cells are also present in the bone marrow, perichondrium, and periosteum. (hc) hypertrophic cartilage. (C and D) PI and Apop staining of 3-week-old (C) and 4-week-old (D) metatarsal growth plates. Arrows in the gelatinase B–null growth plates show apoptotic chondrocytes in the middle of the hypertrophic cartilage at three weeks (C) or in the vascularized and ossified hypertrophic cartilage at 4 weeks (D). Scale bars = 200 μm.
Figure 5. Expression of Gelatinase B at the Growth Plate
(A–D) Bright-field (A and B) and dark-field (C and D) photographs of a section of 3-week-old wild-type metartarsal growth plate hybridized with a gelatinase B antisense mRNA probe. Arrows point to gelatinase B expressing cells at the cartilage–bone junction. (B) and (D) are magnified views of (A) and (C). (ep) epiphysis. Scale bar = 100 μm. (E and F) TRAP staining of an adjacent section (E) and magnified view (F) showing TRAP positive (red) cells at the cartilage–bone junction (white arrows) and on the trabecular bone surfaces (black arrows). Note the distribution of TRAP-positive cells is similar to that of gelatinase B–expressing cells. (G and H) Immunohistochemistry of 3-week-old wild type metatarsal growth plate with an antibody to gelatinase B, showing staining at the cartilage bone juction and trabecular surfaces. Magnified view (H) shows staining of ECM and stellate cells (arrow). (I and J) Immunohistochemistry of 3-week-old wild-type and gelatinase B–null metatarsal growth plates with an antibody to PECAM-1 showing staining of blood vessels at the cartilage–bone junction (white arrows) and in between trabecular bones (black arrows).
Figure 6. Growth Plates of Gelatinase B–Null Mice after Bone Marrow Transplantation
(A) Histological sections of metatarsals from untransplanted and bone marrow (BM) transplanted mice at 3 and 4 weeks after transplantation. Arrow points to area of ectopic ossification in the untransplanted 4-week-old growth plate. Scale bar = 200 μm. (B) TRAP staining and in situ hybridization with an antisense mRNA gelatinase B probe showing that gelatinase B–expressing cells, some of which correspond to TRAP-positive cells, appear at the cartilage–bone junction and on the trabecular bone surfaces after transplantation (arrows).
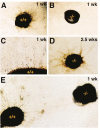
Figure 7. In Vitro Angiogenesis Assay
(A–C) Hypertrophic cartilage from 2-week-old wild-type (A) and gelatinase B–Null (B) mice after one week in a collagen gel coculture with endothelial cells, showing an angiogenic response from the endothelial cells only to wild-type cartilage. Magnified view (C) shows tubular structures formed by endothelial cells surrounding the wild-type hypertrophic cartilage. (D) Gelatinase B–Null hypertrophic cartilage after 2.5 weeks in coculture with endothelial cells, showing an angiogenic response. (E) Wild-type and gelatinase B–Null cartilage after one week side by side in coculture with endothelial cell, showing angiogenic response only to wild-type cartilage that is not abrogated by the presence of the gelatinase B–Null cartilage.
Similar articles
- SOX9 is a major negative regulator of cartilage vascularization, bone marrow formation and endochondral ossification.
Hattori T, Müller C, Gebhard S, Bauer E, Pausch F, Schlund B, Bösl MR, Hess A, Surmann-Schmitt C, von der Mark H, de Crombrugghe B, von der Mark K. Hattori T, et al. Development. 2010 Mar;137(6):901-11. doi: 10.1242/dev.045203. Development. 2010. PMID: 20179096 - Disrupted type II collagenolysis impairs angiogenesis, delays endochondral ossification and initiates aberrant ossification in mouse limbs.
Gauci SJ, Golub SB, Tatarczuch L, Lee E, Chan D, Walsh NC, Little CB, Stanton H, Lokmic Z, Sims NA, Mackie EJ, Fosang AJ. Gauci SJ, et al. Matrix Biol. 2019 Oct;83:77-96. doi: 10.1016/j.matbio.2019.08.001. Epub 2019 Aug 2. Matrix Biol. 2019. PMID: 31381970 - Angiogenesis and bone growth.
Gerber HP, Ferrara N. Gerber HP, et al. Trends Cardiovasc Med. 2000 Jul;10(5):223-8. doi: 10.1016/s1050-1738(00)00074-8. Trends Cardiovasc Med. 2000. PMID: 11282299 Review. - The hypertrophic chondrocyte: To be or not to be.
Hallett SA, Ono W, Ono N. Hallett SA, et al. Histol Histopathol. 2021 Oct;36(10):1021-1036. doi: 10.14670/HH-18-355. Epub 2021 Jun 17. Histol Histopathol. 2021. PMID: 34137454 Free PMC article. Review.
Cited by
- Enocyanin promotes osteogenesis and bone regeneration by inhibiting MMP9.
Mao W, Zheng Y, Zhang W, Yin J, Liu Z, He P, Hou G, Huang G, Chen H, Lin J, Xu J, Li A, Qin S. Mao W, et al. Int J Mol Med. 2025 Jan;55(1):9. doi: 10.3892/ijmm.2024.5450. Epub 2024 Nov 8. Int J Mol Med. 2025. PMID: 39513591 Free PMC article. - The bone Gla protein osteocalcin is expressed in cranial neural crest cells.
Kalev-Altman R, Fraggi-Rankis V, Monsonego-Ornan E, Sela-Donenfeld D. Kalev-Altman R, et al. BMC Res Notes. 2024 Nov 5;17(1):329. doi: 10.1186/s13104-024-06990-7. BMC Res Notes. 2024. PMID: 39501347 Free PMC article. - Deficiencies in corin and atrial natriuretic peptide-mediated signaling impair endochondral ossification in bone development.
Zhou Z, Mao X, Jiang C, Li W, Zhou T, Liu M, Sun S, Wang M, Dong N, Wu Q, Zhou H. Zhou Z, et al. Commun Biol. 2024 Oct 23;7(1):1380. doi: 10.1038/s42003-024-07077-6. Commun Biol. 2024. PMID: 39443661 Free PMC article. - Neutrophil Biomarkers Can Predict Cardiotoxicity of Anthracyclines in Breast Cancer.
Todorova VK, Azhar G, Stone A, Malapati SJ, Che Y, Zhang W, Makhoul I, Wei JY. Todorova VK, et al. Int J Mol Sci. 2024 Sep 9;25(17):9735. doi: 10.3390/ijms25179735. Int J Mol Sci. 2024. PMID: 39273682 Free PMC article. - Converging Mechanisms of Vascular and Cartilaginous Calcification.
Gheorghe SR, Crăciun AM, Ilyés T, Tisa IB, Sur L, Lupan I, Samasca G, Silaghi CN. Gheorghe SR, et al. Biology (Basel). 2024 Jul 26;13(8):565. doi: 10.3390/biology13080565. Biology (Basel). 2024. PMID: 39194503 Free PMC article. Review.
References
- Aharinejad S, Marks SC, Jr., Bock P, MacKay CA, Larson EK, Tahamtani A, Mason-Savas A, Firbas W. Microvascular pattern in the metaphysis during bone growth. Anat. Rec. 1995;242:111–122. - PubMed
- Albrecht U, Eichele G, Helms JA, Lu H. Visualization of gene expression patterns by in situ hybridization. In: Daston GP, editor. Molecular and Cellular Methods in Developmental Toxicology. CRC Press; Boca Raton: 1997. pp. 23–48.
- Alexander CM, Hansell EJ, Behrendtsen O, Flannery ML, Kishnani NS, Hawkes SP, Werb Z. Expression and function of matrix metalloproteinases and their inhibitors at the maternal-embryonic boundary during mouse embryo implantation. Development. 1996;122:1723–1736. - PubMed
- Alini M, Marriott A, Chen T, Abe S, Poole AR. A novel angiogenic molecule produced at the time of chondrocyte hypertrophy during endochondral bone formation. Dev. Biol. 1996;176:124–132. - PubMed
- Amizuka N, Henderson JE, Hoshi K, Warshawsky H, Ozawa H, Goltzman D, Karaplis AC. Programmed cell death of chondrocytes and aberrant chondrogenesis in mice homozygous for parathyroid hormone-related peptide gene deletion. Endocrinology. 1996;137:5055–5067. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA037395/CA/NCI NIH HHS/United States
- R37 CA037395/CA/NCI NIH HHS/United States
- T32 HL007185/HL/NHLBI NIH HHS/United States
- R01 HL047328-08/HL/NHLBI NIH HHS/United States
- R01 HL047328/HL/NHLBI NIH HHS/United States
- R01 CA037395-19/CA/NCI NIH HHS/United States
- T32 HL007185-26/HL/NHLBI NIH HHS/United States
- HD26732/HD/NICHD NIH HHS/United States
- HL47328/HL/NHLBI NIH HHS/United States
- P50 DE010306-05S10005/DE/NIDCR NIH HHS/United States
- DE10306/DE/NIDCR NIH HHS/United States
- P01 HD026732-070003/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases