The development of local, layer-specific visual cortical axons in the absence of extrinsic influences and intrinsic activity - PubMed (original) (raw)
The development of local, layer-specific visual cortical axons in the absence of extrinsic influences and intrinsic activity
J L Dantzker et al. J Neurosci. 1998.
Abstract
The laminar specificity of vertical connections in the primary visual cortex (area 17) develops precisely from the outset, leading to the hypothesis that layer-specific axonal targeting is attributable to molecular cues intrinsic to the cortex (Lund et al., 1977; Katz and Callaway, 1992). However, alternative factors that could influence axonal development have not been investigated. This study examines the roles of intrinsic cortical activity and extrinsic influences that could arise from earlier-formed connections with outside cortical and subcortical areas. Organotypic slice cultures were prepared from ferret area 17 before the formation of local axonal connections and were incubated for 5-7 d to allow initial, local axonal arbors to form in the absence of extrinsic influences. Additionally, some slices were cultured in the presence of the Na+ channel blocker tetrodotoxin to block spontaneous action potentials within the slice. Individual neurons were labeled intracellularly with biocytin, and their patterns of local axonal arborizations were reconstructed. This study focuses on the development of layer 6 pyramidal neurons, the axons of which in vivo bypass an incorrect target, layer 5, before specifically arborizing in their local target, layer 4. We found that axonal arbors developing in vitro preferentially arborized in layer 4 versus layer 5. However, inhibition of spontaneous activity within the cortical slice decreased this specificity, resulting in similar numbers of axonal branches in layers 4 and 5. Thus, although cortical axons do not require influences from outside areas, intrinsic spontaneous activity is required for specific axonal arborization in correct laminar targets.
Figures
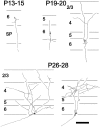
Fig. 1.
Summary of in vivo development of axonal arbors from ferret layer 6 pyramidal neurons. Dendrites are omitted for clarity. At P13–15 layer 6 pyramids have not yet sent an axon collateral into the overlying cortical layers but have a single descending axon extending into the white matter. By_P19–20_, neurons have a few recurrent axon collaterals that are just beginning to form branches in layer 4. Later in development, the density of axonal arbors continues to increase in layers 4 and 2/3, with very few arbors ever forming in layer 5. Figure modified from Callaway and Lieber (1996). Scale bar, 200 μm.
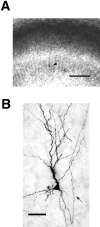
Fig. 2.
Photographs of an intracellularly labeled layer 6 pyramidal neuron in a ferret area 17 slice culture. A, Low-power view illustrating cortical layers revealed by staining with thionin. Layer 5 is distinct as the light band in the_middle_ of the slice. The dark band below it is layer 6, containing a single labeled cell indicated by the_arrowhead_. Layers 2/3 and 4 are not distinct at this stage in development and are referred together as layers 2–4. Slice is oriented with pial surface toward top. Scale bar, 200 μm.B, High-power view showing quality of biocytin labeling and morphology of dendrites and axons that developed in culture. A recurrent axon collateral that originated from the white matter side of the cell body is denoted by the arrow. The small beads on the axon are synaptic swellings (Martin and Whitteridge, 1984), which are evenly spaced along the axon. Scale bar, 50 μm.
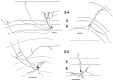
Fig. 3.
Camera lucida drawings illustrating typical axons from layer 6 pyramidal neurons grown in vitro. Dendrites are omitted for clarity. Most cells formed a sparse to moderate density of axonal arbors preferentially in layers 2–4, as exemplified by the cells on the top row and the bottom left quadrant. Usually, these axons were restricted to the region above the cell body, as seen in vivo. Sometimes, an axon collateral would project laterally up to 2 mm away from the cell body before arborizing in the cortical layers (cell in top left quadrant). The cell in the bottom right quadrant_represents a less mature arbor pattern (P14 + 5 dic) seen in five of the 24 cells in the sample. Axons are just beginning to ascend and branch in layer 4. Such cells are very similar to in vivo cells labeled at P19–P20 (Fig. 1). Most cells appeared more mature, with arbor patterns and density similar to the remaining three cells. The solid polygons are cell bodies, and the_fine horizontal lines indicate laminar borders. The most pial laminar border represents the beginning of layer 1. Scale bars, 100 μm.
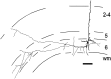
Fig. 4.
Presumptive “claustrum-projecting” layer 6 pyramidal neuron. In cat area 17, such cells have apical dendrites extending into layer 1 and local axonal arbors predominantly in layers 5 and 6 (Katz, 1987). This cell was unique in its preference to arborize in layer 5. Finer neuronal processes indicate axons and thicker ones indicate dendrites. Scale bar, 100 μm.
Fig. 5.
Camera lucida reconstructions of axonal arbors of four representative layer 6 pyramidal neurons cultured in media containing 1 μm TTX. Unlike in standard media cultures, TTX-treated layer 6 pyramids formed as many or more axonal arbors in layer 5 than layers 2–4. This is particularly noticeable for the cells in the_top row_, which have many axonal branches in layer 5 (7 dic). Conventions as in Figure 3. Scale bars, 100 μm.
Fig. 6.
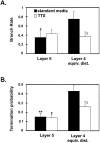
Mean branch rates and termination probabilities in layer 5 and layer 4 ed for cells from standard media (filled bars) and TTX groups (open bars). A, In standard media, the rate at which axon collaterals branch in layer 4 is significantly higher than layer 5, but there is not a difference in TTX. B, For both standard media and TTX groups, the termination probability is significantly higher in layer 4 ed than layer 5. Comparisons of values in layer 4 ed between the standard media and TTX groups show a reduction of the branch rates and termination probabilities in layer 4 ed. §p = 0.06. Significant difference between values in layer 4 ed and layer 5: *p < 0.05; **p < 0.01.
Fig. 7.
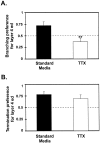
Comparison of arborization preference for layer 4 ed versus layer 5 between standard media and TTX cultures. Preference values were calculated (see Results) so that the relationship between layer 4 ed and layer 5 could be directly compared between the two groups. The dashed line corresponds to no preference for either layer. Values above the line correspond to a preference for layer 4 ed. A, Axons grown in standard media show a significantly greater preference to branch in layer 4 ed than those grown in TTX. B, However, the preference for axons to terminate in layer 4 ed was similar between the groups. **p < 0.01.
Similar articles
- Development of visual cortical axons: layer-specific effects of extrinsic influences and activity blockade.
Butler AK, Dantzker JL, Shah RB, Callaway EM. Butler AK, et al. J Comp Neurol. 2001 Feb 12;430(3):321-31. doi: 10.1002/1096-9861(20010212)430:3<321::aid-cne1033>3.0.co;2-7. J Comp Neurol. 2001. PMID: 11169470 - Reorganization of exuberant axonal arbors contributes to the development of laminar specificity in ferret visual cortex.
Borrell V, Callaway EM. Borrell V, et al. J Neurosci. 2002 Aug 1;22(15):6682-95. doi: 10.1523/JNEUROSCI.22-15-06682.2002. J Neurosci. 2002. PMID: 12151547 Free PMC article. - Development of axonal arbors of layer 6 pyramidal neurons in ferret primary visual cortex.
Callaway EM, Lieber JL. Callaway EM, et al. J Comp Neurol. 1996 Dec 9;376(2):295-305. doi: 10.1002/(SICI)1096-9861(19961209)376:2<295::AID-CNE10>3.0.CO;2-L. J Comp Neurol. 1996. PMID: 8951644 - Cell type specificity of local cortical connections.
Callaway EM. Callaway EM. J Neurocytol. 2002 Mar-Jun;31(3-5):231-7. doi: 10.1023/a:1024165824469. J Neurocytol. 2002. PMID: 12815242 Review. - Specification of layer-specific connections in the developing cortex.
Bolz J, Castellani V, Mann F, Henke-Fahle S. Bolz J, et al. Prog Brain Res. 1996;108:41-54. doi: 10.1016/s0079-6123(08)62531-5. Prog Brain Res. 1996. PMID: 8979793 Review.
Cited by
- Neural Signaling in Cancer.
Keough MB, Monje M. Keough MB, et al. Annu Rev Neurosci. 2022 Jul 8;45:199-221. doi: 10.1146/annurev-neuro-111020-092702. Epub 2022 Mar 8. Annu Rev Neurosci. 2022. PMID: 35259916 Free PMC article. Review. - The neuroscience of cancer.
Mancusi R, Monje M. Mancusi R, et al. Nature. 2023 Jun;618(7965):467-479. doi: 10.1038/s41586-023-05968-y. Epub 2023 Jun 14. Nature. 2023. PMID: 37316719 Free PMC article. Review. - Inhibitory mechanism by polysialic acid for lamina-specific branch formation of thalamocortical axons.
Yamamoto N, Inui K, Matsuyama Y, Harada A, Hanamura K, Murakami F, Ruthazer ES, Rutishauser U, Seki T. Yamamoto N, et al. J Neurosci. 2000 Dec 15;20(24):9145-51. doi: 10.1523/JNEUROSCI.20-24-09145.2000. J Neurosci. 2000. PMID: 11124992 Free PMC article. - Neocortical axon arbors trade-off material and conduction delay conservation.
Budd JM, Kovács K, Ferecskó AS, Buzás P, Eysel UT, Kisvárday ZF. Budd JM, et al. PLoS Comput Biol. 2010 Mar 12;6(3):e1000711. doi: 10.1371/journal.pcbi.1000711. PLoS Comput Biol. 2010. PMID: 20300651 Free PMC article. - Netrin-4 regulates thalamocortical axon branching in an activity-dependent fashion.
Hayano Y, Sasaki K, Ohmura N, Takemoto M, Maeda Y, Yamashita T, Hata Y, Kitada K, Yamamoto N. Hayano Y, et al. Proc Natl Acad Sci U S A. 2014 Oct 21;111(42):15226-31. doi: 10.1073/pnas.1402095111. Epub 2014 Oct 6. Proc Natl Acad Sci U S A. 2014. PMID: 25288737 Free PMC article.
References
- Annis CM, Robertson RT, O’Dowd DK. Aspects of early postnatal development of cortical neurons that proceed independently of normally present extrinsic influences. J Neurobiol. 1993;24:1460–1480. - PubMed
- Aoyagi A, Nishikawa K, Saito H, Abe K. Characterization of basic fibroblast growth factor-mediated acceleration of axonal branching in cultured rat hippocampal neurons. Brain Res. 1994;661:117–126. - PubMed
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole cell recording from neurons in slices of reptilian and mammalian cerebral cortex. J Neurosci Methods. 1989;30:203–210. - PubMed
- Blochl A, Thoenen H. Characterization of nerve growth factor (NGF) release from hippocampal neurons: evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci. 1995;7:1220–1228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources