Rapsyn clusters neuronal acetylcholine receptors but is inessential for formation of an interneuronal cholinergic synapse - PubMed (original) (raw)
Rapsyn clusters neuronal acetylcholine receptors but is inessential for formation of an interneuronal cholinergic synapse
G Feng et al. J Neurosci. 1998.
Abstract
Nicotinic acetylcholine receptors (AChRs) are clustered at high density in the postsynaptic membranes of skeletal neuromuscular junctions and cholinergic interneuronal synapses. A cytoplasmic protein, rapsyn, is essential for AChR clustering in muscle. Here, we asked whether rapsyn mediates neuronal AChR clustering at cholinergic synapses in a mammalian sympathetic ganglion, the superior cervical ganglion (SCG). Several observations supported this possibility: (1) AChR clusters containing the alpha3-5 and beta2 subunits, homologs of the muscle AChR subunits, are present at SCG synapses; (2) rapsyn RNA is readily detectable in the SCG; and (3) expression of recombinant rapsyn in heterologous cells induces aggregation of coexpressed neuronal AChR subunits. However, rapsyn protein was undetectable at ganglionic synaptic sites. Moreover, aggregates of neuronal AChRs induced in heterologous cells by full-length rapsyn remained intracellular, whereas rapsyn-induced clusters of muscle AChRs reached the cell surface. Additional studies revealed a second rapsyn RNA species in SCG generated by alternative splicing and competent to encode a novel short rapsyn isoform. However, this isoform clustered neither neuronal nor muscle AChRs in heterologous cells. Most telling, the number, size, and density of AChR clusters in SCG did not differ significantly between neonatal mice bearing a targeted mutation of the rapsyn gene and littermate controls. Thus, rapsyn is dispensable for clustering of ganglionic neuronal nicotinic AChRs.
Figures
Fig. 1.
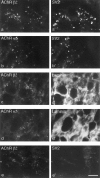
Distribution of AChRs in mouse SCG. Sections of P23–P25 SCG were double-stained with antibodies to neuronal AChR subunits (a–e) plus anti-SV2 (a′,b′, e′) or anti-laminin (c′, d′). Both anti-AChR β2 (mAb 270;a, c, e) and anti-AChR α5 (mAb 210; b, d) stained small discrete patches, most of which were associated with nerve terminals (a′, b′) and were clustered in the neuropil rather than on the surface of somata (c′, d′). The section in _e_and e′ was from a ganglion that had been denervated 3 d earlier; SV2-positive nerve terminals had degenerated but AChR clusters persisted, demonstrating that most, if not all, AChRs are postsynaptic. Scale bar (in e′): a, b, 10 μm; c–e, 20 μm.
Fig. 2.
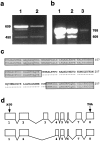
Detection and characterization of rapsyn RNA.a, RT-PCR of total RNA from muscle (lane 1) and SCG (lane 2), using rapsyn-specific primers. A band of the predicted size of 609 nt was readily amplified from both samples but was more abundant in muscle than in SCG. A band of 450 nt was also detected in both samples but was a larger fraction of the total product in SCG. b, RT-PCR of poly(A+) RNA from muscle (lane 1), PC12 cells (lane 2), and brain (lane 3) using a second set of rapsyn-specific primers designed to amplify a 768 nt segment. Low levels of rapsyn RNA were detected in the brain sample, and relatively high levels were detected in PC12 cells. As in_a_, a band ∼150 nt smaller than the predicted band was also amplified. c, Part of the sequence of the 450 nt band from a, lane 2 (bottom line), aligned with sequence of full-length rapsyn (top line). The shorter band encodes a protein that lacks a 53-amino acid segment. d, The short form of rapsyn is likely to be generated from an alternatively spliced mRNA that lacks exon 3.Top, structure of the rapsyn gene, from Gautam et al. (1994). Bottom, The alternatively spliced RNA that encodes the short form.
Fig. 3.
Rapsyn protein is undetectable at AChR clusters in SCG. Sections of mouse skeletal muscle (a–c) or SCG (d–f) were labeled with mAb 210, which recognizes the muscle AChR α subunit and the neuronal AChR α5 subunit (a, d), plus either polyclonal antibodies to the N terminus of rapsyn (b,e) or a monoclonal antibody to the C terminus of rapsyn (c, f). Concentrations were adjusted so that staining was more intense by anti-rapsyn than by anti-AChR in muscle. Although muscle and SCG were stained under identical conditions, no staining by anti-rapsyn was detectable in SCG. Thus, if rapsyn is present at synaptic sites in SCG, it is not present at the 1:1 ratio with AChRs found in muscle. Because mAb 210 stains all AChRs in muscle but only a subset of AChRs in SCG, the difference in the AChR/rapsyn ratio between the two tissues is even greater than it appears to be in these micrographs. Scale bar (in_f_): a–c, 50 μm;d–f, 6 μm.
Fig. 4.
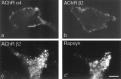
Rapsyn-induced clustering of AChRs in transiently transfected cells. QT-6 cells were transfected with expression vectors encoding the AChR β2 (a, e,i, m), α3 plus β2 (b,f, j, n), α4 plus β2 (c, g, k,o), or α plus β plus γ plus δ (d,h, l, p) subunits, either without (a–h) or with (i–p) an expression vector encoding mouse rapsyn. Two days later, the cultures were stained with antibodies to the AChR β2 (a–c,e–g, i–k, m–o) or α subunit (d, h, l,p), either without (NP) or after permeabilization (P) to reveal cell surface or all AChRs, respectively. Cultures in m–p were double-stained with anti-rapsyn (m′–p′). Rapsyn induces clustering of muscle and neuronal receptors, but clusters of neuronal AChRs are retained intracellularly. Scale bar, 10 μm.
Fig. 5.
Rapsyn-induced clustering of AChRs in stably transfected human cells. HEK 293 cells stably transfected with AChR α4 plus β2 expression vectors were stained with antibodies to α4 (a) or β2 (b). AChRs were diffusely distributed on the cell surface. Sister cultures were transiently transfected with rapsyn and then permeabilized and double-stained with anti-β2 (c) and anti-rapsyn (c′). Rapsyn induced clustering of AChRs. No clusters were detected in nonpermeabilized cells (data not shown), indicating that in HEK 293 cells as in QT-6 cells, clusters of neuronal AChRs are retained intracellularly. Scale bar, 10 μm.
Fig. 6.
The short isoform of rapsyn cannot cluster AChRs. QT-6 cells were transiently transfected with an expression vector encoding the short form of rapsyn shown in Figure 2_c_, in addition to AChR α plus β plus γ plus δ (a), α3 plus β2 (b), or α4 plus β2 (c) subunits. Two days later, cells were permeabilized and stained with anti-AChR (a–c) plus anti-rapsyn (a′–c′). Antibodies to both the C terminus (a′–c′) and N terminus (data not shown) (Fig. 3) recognized the short isoform. Both AChRs and the short form of rapsyn were diffusely distributed. Scale bar, 10 μm.
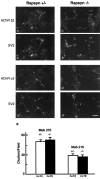
Fig. 7.
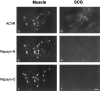
AChR clusters in SCG of rapsyn−/− mice. Sections of SCG from E18 rapsyn+/− (a, c) or rapsyn−/− (b,d) littermates were double-stained with anti-AChR β2 (a, b) or α5 (c,d) plus anti-SV2 (a′–d′). AChRs clusters were present at both synaptic and extrasynaptic sites in perinatal ganglia from both mutants and controls. e, Numbers of AChR-rich clusters in mutant and control ganglia. Scale bar, 10 μm.
Fig. 8.
Immunostaining of gephyrin in brain and SCG. Sections of control adult brain (a), control E18 SCG (b), and rapsyn−/− SCG (c) were stained with anti-gephyrin. Gephyrin showed punctate staining in brain but no staining in SCG. Scale bar, 10 μm.
Similar articles
- Rapsyn facilitates recovery from desensitization in fetal and adult acetylcholine receptors expressed in a muscle cell line.
Cetin H, Liu W, Cheung J, Cossins J, Vanhaesebrouck A, Maxwell S, Vincent A, Beeson D, Webster R. Cetin H, et al. J Physiol. 2019 Jul;597(14):3713-3725. doi: 10.1113/JP277819. Epub 2019 Jun 17. J Physiol. 2019. PMID: 31158924 Free PMC article. - Neural agrin increases postsynaptic ACh receptor packing by elevating rapsyn protein at the mouse neuromuscular synapse.
Brockhausen J, Cole RN, Gervásio OL, Ngo ST, Noakes PG, Phillips WD. Brockhausen J, et al. Dev Neurobiol. 2008 Aug;68(9):1153-69. doi: 10.1002/dneu.20654. Dev Neurobiol. 2008. PMID: 18506821 - The synapse-associated protein rapsyn regulates tyrosine phosphorylation of proteins colocalized at nicotinic acetylcholine receptor clusters.
Qu Z, Apel ED, Doherty CA, Hoffman PW, Merlie JP, Huganir RL. Qu Z, et al. Mol Cell Neurosci. 1996;8(2-3):171-84. doi: 10.1006/mcne.1996.0055. Mol Cell Neurosci. 1996. PMID: 8918833 - Clustering of nicotinic acetylcholine receptors: from the neuromuscular junction to interneuronal synapses.
Huh KH, Fuhrer C. Huh KH, et al. Mol Neurobiol. 2002 Feb;25(1):79-112. doi: 10.1385/MN:25:1:079. Mol Neurobiol. 2002. PMID: 11890459 Review. - The postsynaptic submembrane machinery at the neuromuscular junction: requirement for rapsyn and the utrophin/dystrophin-associated complex.
Banks GB, Fuhrer C, Adams ME, Froehner SC. Banks GB, et al. J Neurocytol. 2003 Jun-Sep;32(5-8):709-26. doi: 10.1023/B:NEUR.0000020619.24681.2b. J Neurocytol. 2003. PMID: 15034263 Review.
Cited by
- A Flow Cytometric Assay to Detect Functional Ganglionic Acetylcholine Receptor Antibodies by Immunomodulation in Autoimmune Autonomic Ganglionopathy.
Urriola N, Spies JM, Blazek K, Lang B, Adelstein S. Urriola N, et al. Front Immunol. 2021 Jun 23;12:705292. doi: 10.3389/fimmu.2021.705292. eCollection 2021. Front Immunol. 2021. PMID: 34249013 Free PMC article. - Main immunogenic region structure promotes binding of conformation-dependent myasthenia gravis autoantibodies, nicotinic acetylcholine receptor conformation maturation, and agonist sensitivity.
Luo J, Taylor P, Losen M, de Baets MH, Shelton GD, Lindstrom J. Luo J, et al. J Neurosci. 2009 Nov 4;29(44):13898-908. doi: 10.1523/JNEUROSCI.2833-09.2009. J Neurosci. 2009. PMID: 19890000 Free PMC article. - Nicotinic acetylcholine receptor is internalized via a Rac-dependent, dynamin-independent endocytic pathway.
Kumari S, Borroni V, Chaudhry A, Chanda B, Massol R, Mayor S, Barrantes FJ. Kumari S, et al. J Cell Biol. 2008 Jun 30;181(7):1179-93. doi: 10.1083/jcb.200709086. J Cell Biol. 2008. PMID: 18591431 Free PMC article. - Alpha7 nicotinic acetylcholine receptors occur at postsynaptic densities of AMPA receptor-positive and -negative excitatory synapses in rat sensory cortex.
Levy RB, Aoki C. Levy RB, et al. J Neurosci. 2002 Jun 15;22(12):5001-15. doi: 10.1523/JNEUROSCI.22-12-05001.2002. J Neurosci. 2002. PMID: 12077196 Free PMC article. - Cytoskeletal links of neuronal acetylcholine receptors containing alpha 7 subunits.
Shoop RD, Yamada N, Berg DK. Shoop RD, et al. J Neurosci. 2000 Jun 1;20(11):4021-9. doi: 10.1523/JNEUROSCI.20-11-04021.2000. J Neurosci. 2000. PMID: 10818137 Free PMC article.
References
- Apel ED, Roberds SL, Campbell KP, Merlie JP. Rapsyn may function as a link between the acetylcholine receptor and the agrin-binding dystrophin-associated glycoprotein complex. Neuron. 1995;15:115–126. - PubMed
- Apel ED, Glass DJ, Moscoso LM, Yancopoulos GD, Sanes JR. Rapsyn is required for MuSK signaling and recruits synaptic components to a MuSK-containing scaffold. Neuron. 1997;18:623–635. - PubMed
- Boulter J, Evans K, Goldman D, Martin G, Treco D, Heinemann S, Patrick J. Isolation of a cDNA clone coding for a possible neural nicotinic acetylcholine receptor alpha-subunit. Nature. 1986;319:368–374. - PubMed
- Brakeman PR, Lanahan AA, O’Brien R, Roche K, Barnes CA, Huganir RL, Worley PF. Homer: a protein that selectively binds metabotropic glutamate receptors. Nature. 1997;386:284–288. - PubMed
- Brennan C, Scotland PB, Froehner SC, Henderson LP. Functional properties of acetylcholine receptors coexpressed with the 43K protein in heterologous cell systems. Dev Biol. 1992;149:100–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous