Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection - PubMed (original) (raw)
Levofloxacin population pharmacokinetics and creation of a demographic model for prediction of individual drug clearance in patients with serious community-acquired infection
S L Preston et al. Antimicrob Agents Chemother. 1998 May.
Abstract
Population pharmacokinetic modeling is a useful approach to obtaining estimates of both population and individual pharmacokinetic parameter values. The potential for relating pharmacokinetic parameters to pharmacodynamic outcome variables, such as efficacy and toxicity, exists. A logistic regression relationship between the probability of a successful clinical and microbiological outcome and the peak concentration-to-MIC ratio (and also the area under the plasma concentration-time curve [AUC]/MIC ratio) has previously been developed for levofloxacin; however, levofloxacin assays for determination of the concentration in plasma are not readily available. We attempted to derive and validate demographic variable models to allow prediction of the peak concentration in plasma and clearance (CL) from plasma for levofloxacin. Two hundred seventy-two patients received levofloxacin intravenously for the treatment of community-acquired infection of the respiratory tract, skin or soft tissue, or urinary tract, and concentrations in plasma, guided by optimal sampling theory, were obtained. Patient data were analyzed by the Non-Parametric Expectation Maximization approach. Maximum a posteriori probability Bayesian estimation was used to generate individual parameter values, including CL. Peak concentrations were simulated from these estimates. The first 172 patients were used to produce demographic models for the prediction of CL and the peak concentration. The remaining 100 patients served as the validation group for the model. A median bias and median precision were calculated. A two-compartment model was used for the population pharmacokinetic analysis. The mean CL and the mean volume of distribution of the central compartment (V1) were 9.27 liters/h and 0.836 liter/kg, respectively. The mean values for the intercompartmental rate constants, the rate constant from the central compartment to the peripheral compartment (Kcp) and the rate constant from the peripheral compartment to the central compartment (Kpc), were 0.487 and 0.647 h(-1), respectively. The mean peak concentration and the mean AUC values normalized to a dosage of 500 mg every 24 h were 8.67 microg/ml and 72.53 microg x h/ml, respectively. The variables included in the final model for the prediction of CL were creatinine clearance (CLCR), race, and age. The median bias and median precision were 0.5 and 18.3%, respectively. Peak concentrations were predicted by using the demographic model-predicted parameters of CL, V1, Kcp and Kpc, in the simulation. The median bias and the median precision were 3.3 and 21.8%, respectively. A population model of the disposition of levofloxacin has been developed. Population demographic models for the prediction of peak concentration and CL from plasma have also been successfully developed. However, the performance of the model for the prediction of peak concentration was likely insufficient to be of adequate clinical utility. The model for the prediction of CL was relatively robust, with acceptable bias and precision, and explained a reasonable amount of the variance in the CL of levofloxacin from plasma in the population (r2 = 0.396). Estimated CLCR, age, and race were the final model covariates, with CLCR explaining most of the population variance in the CL of levofloxacin from plasma. This model can potentially optimize the benefit derived from the pharmacodynamic relationships previously developed for levofloxacin.
Figures
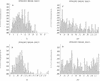
FIG. 1
Probability distributions of pharmacokinetic parameters in the population. The marginal distributions, which indicate the probability of occurrence of pharmacokinetic parameter values in the population, are displayed. (A) Slope of _V_1 to body weight (VS; in liters/kilogram). (B) CL (in liters/hour). (C and D) _K_cp (KCP) and _K_pc (KPC), (in hours−1), respectively.
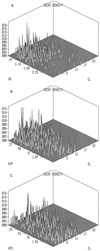
FIG. 2
Three-dimensional probability distributions of pharmacokinetic parameters. (A to C) Three-dimensional probability distribution plots of the population for pharmacokinetic parameters. Parameters and units are as described in the legend to Fig. 1.
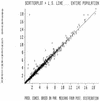
FIG. 3
Observed versus MAP Bayesian-predicted concentrations in 272 patients. A scatter plot of observed versus MAP Bayesian-predicted concentrations based on pharmacokinetic parameter medians is shown. The slope and the intercept of the line are 1.01 and 0.0054, respectively. The slope is not significantly different from 1.0, and the intercept is not significantly different from 0.0. The _r_2 value is 0.966.
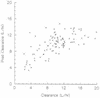
FIG. 4
Measured versus predicted CL obtained with the demographic model. The measured versus predicted CL for the validation group (n = 100) obtained with the demographic model developed in this study is shown. Note that when the measured CL reaches approximately 13 liters/h, the predictive performance decreases (the model underpredicts CL).
Similar articles
- Population pharmacokinetics of oral levofloxacin 500 mg once-daily dosage in community-acquired lower respiratory tract infections: results of a prospective multicenter study in China.
Zhang J, Xu JF, Liu YB, Xiao ZK, Huang JA, Si B, Sun SH, Xia QM, Wu XJ, Cao GY, Shi YG, Zhang YY. Zhang J, et al. J Infect Chemother. 2009 Oct;15(5):293-300. doi: 10.1007/s10156-009-0714-8. Epub 2009 Oct 24. J Infect Chemother. 2009. PMID: 19856067 Clinical Trial. - Double-blind evaluation of the safety and pharmacokinetics of multiple oral once-daily 750-milligram and 1-gram doses of levofloxacin in healthy volunteers.
Chien SC, Wong FA, Fowler CL, Callery-D'Amico SV, Williams RR, Nayak R, Chow AT. Chien SC, et al. Antimicrob Agents Chemother. 1998 Apr;42(4):885-8. doi: 10.1128/AAC.42.4.885. Antimicrob Agents Chemother. 1998. PMID: 9559801 Free PMC article. Clinical Trial. - The clinical pharmacokinetics of levofloxacin.
Fish DN, Chow AT. Fish DN, et al. Clin Pharmacokinet. 1997 Feb;32(2):101-19. doi: 10.2165/00003088-199732020-00002. Clin Pharmacokinet. 1997. PMID: 9068926 Review. - Pharmacodynamics of levofloxacin: a new paradigm for early clinical trials.
Preston SL, Drusano GL, Berman AL, Fowler CL, Chow AT, Dornseif B, Reichl V, Natarajan J, Corrado M. Preston SL, et al. JAMA. 1998 Jan 14;279(2):125-9. doi: 10.1001/jama.279.2.125. JAMA. 1998. PMID: 9440662 Clinical Trial. - Population pharmacokinetics of therapeutic monoclonal antibodies.
Dirks NL, Meibohm B. Dirks NL, et al. Clin Pharmacokinet. 2010 Oct;49(10):633-59. doi: 10.2165/11535960-000000000-00000. Clin Pharmacokinet. 2010. PMID: 20818831 Review.
Cited by
- Applications of pharmacometrics in the clinical development and pharmacotherapy of anti-infectives.
Trivedi A, Lee RE, Meibohm B. Trivedi A, et al. Expert Rev Clin Pharmacol. 2013 Mar;6(2):159-70. doi: 10.1586/ecp.13.6. Expert Rev Clin Pharmacol. 2013. PMID: 23473593 Free PMC article. Review. - A one-year prospective study of the safety, tolerability and pharmacokinetics of the highest available dose of paliperidone palmitate in patients with schizophrenia.
Coppola D, Liu Y, Gopal S, Remmerie B, Samtani MN, Hough DW, Nuamah I, Sulaiman A, Pandina G. Coppola D, et al. BMC Psychiatry. 2012 Mar 28;12:26. doi: 10.1186/1471-244X-12-26. BMC Psychiatry. 2012. PMID: 22455454 Free PMC article. Clinical Trial. - Non-Adherence in Adult Male Patients with Community-Acquired Pneumonia: Relative Forgiveness of Amoxicillin versus Respiratory Fluoroquinolones.
Carral N, Lukas JC, Estradé O, Jauregizar N, Morillas H, Suárez E. Carral N, et al. Antibiotics (Basel). 2023 May 1;12(5):838. doi: 10.3390/antibiotics12050838. Antibiotics (Basel). 2023. PMID: 37237741 Free PMC article. - Comparison of levofloxacin, alatrofloxacin, and vancomycin for prophylaxis and treatment of experimental foreign-body-associated infection by methicillin-resistant Staphylococcus aureus.
Vaudaux P, Francois P, Bisognano C, Schrenzel J, Lew DP. Vaudaux P, et al. Antimicrob Agents Chemother. 2002 May;46(5):1503-9. doi: 10.1128/AAC.46.5.1503-1509.2002. Antimicrob Agents Chemother. 2002. PMID: 11959588 Free PMC article. - Mutant prevention concentrations of levofloxacin, pazufloxacin and ciprofloxacin for A. baumannii and mutations in gyrA and parC genes.
Sun C, Hao J, Dou M, Gong Y. Sun C, et al. J Antibiot (Tokyo). 2015 May;68(5):313-7. doi: 10.1038/ja.2014.150. Epub 2014 Nov 5. J Antibiot (Tokyo). 2015. PMID: 25351948
References
- Anonymous. HPLC assays for the stereospecific determination of levofloxacin in human plasma and urine. Data on file. The R. W. Johnson Pharmaceutical Research Institute, Raritan, N.J.
- Cockcroft D W, Gault M H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. - PubMed
- D’Argenio D Z, Schumitzky A. ADAPT II. A program for simulation, identification, and optimal experimental design. User manual. Biomedical Simulations Resource. Los Angeles: University of Southern California; 1992.
- Drusano G L. Optimal sampling theory and population modeling: application to determination of the influence of the microgravity environment on drug distribution and elimination. J Clin Pharmacol. 1991;31:962–967. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources