Effects of toxin A from Clostridium difficile on mast cell activation and survival - PubMed (original) (raw)
Effects of toxin A from Clostridium difficile on mast cell activation and survival
G M Calderón et al. Infect Immun. 1998 Jun.
Abstract
Toxins A and B from Clostridium difficile are the main cause of antibiotic-associated diarrhea and pseudomembranous colitis. They cause fluid accumulation, necrosis, and a strong inflammatory response when inoculated in intestinal loops. Since mast cells are a rich source of inflammatory mediators, abundant in the gut, and known to be involved in C. difficile-induced enteritis, we studied the in vitro effect of toxin A on isolated mast cells. Normal rats sensitized by infection with Nippostrongilus brasiliensis were used to isolate peritoneal mast cells (PMC). PMC from naive rats were stimulated with calcium ionophore A23187 as a model of antigen-independent activation, and PMC from sensitized rats were stimulated with N. brasiliensis antigens to study immunoglobulin E-dependent mast cell activation. After 4 h, toxin A did not induce release of nitric oxide or histamine in naive PMC. However, 10 ng of toxin per ml caused a significant release of tumor necrosis factor alpha (TNF-alpha). In contrast, 1 microg of toxin per ml inhibited antigen or A23187-induced histamine release by PMC. Toxin A at 1 microg/ml for 4 h caused disruption of actin which aggregated in the cytoplasm and around the nucleus. After 24 h, chromatin condensation, cytoplasmic blebbing, and apoptotic-like vesicles were observed; DNA fragmentation was documented also. These results suggest that mast cells may participate in the initial inflammatory response to C. difficile infection by releasing TNF-alpha upon interaction with toxin A. However, longer exposure to toxin A affects the release of inflammatory mediators, perhaps because of the alteration of the cytoskeleton and induction of apoptosis. The impaired functions and survival of mast cells by C. difficile toxin A could hamper the capacity of these cells to counteract the infection, thus prolonging the pathogenic effects of C. difficile toxins.
Figures
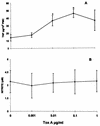
FIG. 1
(A) Effects of toxin A (Tox A) on TNFα production by rat PMC. Cells were incubated with toxin A for 4 h, washed, and then cultured for an additional 6 h before the supernatants were assayed for TNF-α or NO. Results are the means ± SE for five experiments. Shown are effects on TNF-α production (∗, P < 0.05 by comparison with cells not treated with toxin A). (B) Effect on NO production. PMC were treated under same conditions as above (_P_ > 0.05 by comparison with cells not treated with toxin A).
FIG. 2
Effect of C. difficile toxin A on NbAg and on A23187 induction of histamine release by PMC. The cells were incubated for 4 h with different concentrations of toxin A (Tox) and further stimulated with NbAg (Ag; 10 worm equivalents/ml) or A23187 (Ion; 2.5 μM) for 20 min. A significant inhibition (∗, P < 0.01) was observed on mast cells treated with 1 μg of toxin A per ml plus antigen (77%) or toxin A plus A23187 (41%). Results are the mean ± SE of five experiments with duplicates in each experiment.
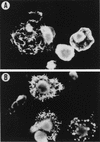
FIG. 3
Effect of toxin A on PMC actin microfilaments. (A) Mast cells showed a normal distribution of actin filaments without toxin A treatment. (B) Mast cells treated with 1 μg of toxin A per ml showed cytoskeletal rearrangements with actin-forming dense deposits in the cytoplasm and cytoplasm retraction.
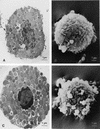
FIG. 4
TEM and SEM of control PMC (A and B) and PMC treated with 1 μg of toxin A per ml for 4 h (C and D). TEM showed that mast cells exposed to toxin A underwent loss of membrane microvilli and nuclear chromatin condensation (C). With SEM, some cells showed signs of alteration: surface blebbing, loss of rounding and normal granule organization, cell membrane disorganization, and changes in morphology (D).
FIG. 5
Analysis of DNA fragmentation of PMC treated with 1 μg of toxin A per ml for 4 h. Ten micrograms of DNA was separated in a 1% agarose gel and stained with SYBR green. Lane A, molecular weight marker profile from a 123-bp ladder from Gibco-BRL; lane B, DNA from PMC incubated in RPMI 1640–5% FBS for 24 h; lane C, fragmented genomic DNA from PMC treated with toxin A for 24 h.
Similar articles
- Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: role of interleukin-1beta, tumor necrosis factor alpha, and leukotrienes.
Rocha MF, Maia ME, Bezerra LR, Lyerly DM, Guerrant RL, Ribeiro RA, Lima AA. Rocha MF, et al. Infect Immun. 1997 Jul;65(7):2740-6. doi: 10.1128/iai.65.7.2740-2746.1997. Infect Immun. 1997. PMID: 9199444 Free PMC article. - Microbes and microbial toxins: paradigms for microbial-mucosal interactions II. The integrated response of the intestine to Clostridium difficile toxins.
Pothoulakis C, Lamont JT. Pothoulakis C, et al. Am J Physiol Gastrointest Liver Physiol. 2001 Feb;280(2):G178-83. doi: 10.1152/ajpgi.2001.280.2.G178. Am J Physiol Gastrointest Liver Physiol. 2001. PMID: 11208538 Review. - Theodore E. Woodward Award. How bacterial enterotoxins work: insights from in vivo studies.
Lamont JT. Lamont JT. Trans Am Clin Climatol Assoc. 2002;113:167-80; discussion 180-1. Trans Am Clin Climatol Assoc. 2002. PMID: 12053708 Free PMC article. Review. - Effects of Clostridium difficile toxin B on activation of rat peritoneal mast cells.
Wex CB, Koch G, Aktories K. Wex CB, et al. Naunyn Schmiedebergs Arch Pharmacol. 1997 Mar;355(3):328-34. doi: 10.1007/pl00004950. Naunyn Schmiedebergs Arch Pharmacol. 1997. PMID: 9089662 - Stem cell factor potentiates histamine secretion by multiple mechanisms, but does not affect tumour necrosis factor-alpha release from rat mast cells.
Lin TJ, Bissonnette EY, Hirsh A, Befus AD. Lin TJ, et al. Immunology. 1996 Oct;89(2):301-7. doi: 10.1046/j.1365-2567.1996.d01-733.x. Immunology. 1996. PMID: 8943730 Free PMC article.
Cited by
- Loratadine as an Anti-inflammatory Agent Against Clostridium difficile Toxin B.
Xie Y, Irwin S, Chupina Estrada A, Nelson B, Bullock A, Fontenot L, Feng H, Sun M, Koon HW. Xie Y, et al. J Infect Dis. 2024 Sep 23;230(3):545-557. doi: 10.1093/infdis/jiae021. J Infect Dis. 2024. PMID: 38243838 - The role of toxins in Clostridium difficile infection.
Chandrasekaran R, Lacy DB. Chandrasekaran R, et al. FEMS Microbiol Rev. 2017 Nov 1;41(6):723-750. doi: 10.1093/femsre/fux048. FEMS Microbiol Rev. 2017. PMID: 29048477 Free PMC article. Review. - High temporal resolution of glucosyltransferase dependent and independent effects of Clostridium difficile toxins across multiple cell types.
D'Auria KM, Bloom MJ, Reyes Y, Gray MC, van Opstal EJ, Papin JA, Hewlett EL. D'Auria KM, et al. BMC Microbiol. 2015 Feb 4;15(1):7. doi: 10.1186/s12866-015-0361-4. BMC Microbiol. 2015. PMID: 25648517 Free PMC article. - The roles of host and pathogen factors and the innate immune response in the pathogenesis of Clostridium difficile infection.
Sun X, Hirota SA. Sun X, et al. Mol Immunol. 2015 Feb;63(2):193-202. doi: 10.1016/j.molimm.2014.09.005. Epub 2014 Sep 18. Mol Immunol. 2015. PMID: 25242213 Free PMC article. Review. - Down-regulation of interleukin-16 in human mast cells HMC-1 by Clostridium difficile toxins A and B.
Gerhard R, Queisser S, Tatge H, Meyer G, Dittrich-Breiholz O, Kracht M, Feng H, Just I. Gerhard R, et al. Naunyn Schmiedebergs Arch Pharmacol. 2011 Mar;383(3):285-95. doi: 10.1007/s00210-010-0592-8. Epub 2011 Jan 26. Naunyn Schmiedebergs Arch Pharmacol. 2011. PMID: 21267712
References
- Bartlett, J. G. 1994. Clostridium difficile: history of its role as an enteric pathogen and the current state of knowledge about the organism. Clin. Infect. Dis. 18(Suppl. 4):S265–S272. - PubMed
- Befus A D, Pearce F L, Gauldie J, Horsewood P, Bienenstock J. Mucosal mast cells. I. Isolation and functional characteristics of rat intestinal mast cells. J Immunol. 1982;128:2475–2480. - PubMed
- Benyon R C, Enciso J A, Befus A D. Analysis of human skin mast cell proteins by two-dimensional gel electrophoresis: identification of tryptase as a siaylated glycoprotein. J Immunol. 1993;151:2699–2706. - PubMed
- Bissonnette E Y, Enciso J A, Befus A D. TGF-β1 inhibits the release of histamine and tumor necrosis factor-α from mast cells through an autocrine pathway. Am J Respir Cell Mol Biol. 1997;16:275–282. - PubMed
- Bissonnette E Y, Hogaboam C M, Wallace J L, Befus A D. Potentiation of tumor necrosis factor-alpha-mediated cytotoxicity of mast cells by their production of nitric oxide. J Immunol. 1991;147:3060–3065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources