The central structural feature of the membrane fusion protein subunit from the Ebola virus glycoprotein is a long triple-stranded coiled coil - PubMed (original) (raw)
The central structural feature of the membrane fusion protein subunit from the Ebola virus glycoprotein is a long triple-stranded coiled coil
W Weissenhorn et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The ectodomain of the Ebola virus Gp2 glycoprotein was solubilized with a trimeric, isoleucine zipper derived from GCN4 (pIIGCN4) in place of the hydrophobic fusion peptide at the N terminus. This chimeric molecule forms a trimeric, highly alpha-helical, and very thermostable molecule, as determined by chemical crosslinking and circular dichroism. Electron microscopy indicates that Gp2 folds into a rod-like structure like influenza HA2 and HIV-1 gp41, providing further evidence that viral fusion proteins from diverse families such as Orthomyxoviridae (Influenza), Retroviridae (HIV-1), and Filoviridae (Ebola) share common structural features, and suggesting a common membrane fusion mechanism.
Figures
Figure 1
(a) Primary structure of the Ebola virus Gp. The numbers indicate amino acid positions (including the signal peptide) for (i) potential carbohydrate sites (4), (ii) the cleavage of Gp into Gp1 and Gp2 occurring at position 501 (5, 6); and (iii) Gp2 anchoring in the membrane by amino acids 651–672. The sequence of the expressed construct pIIGp2(552–650) is shown with the a and d heptad positions of pIIGCN4 in-frame with the predicted a and d positions of the Ebola virus TM protein (14). The cysteines (601 and 608) involved in disulfide formation are indicated and those changed to Ser (556 and 609) are indicated by S. (b) Chemical crosslinking of pIIGp2(552–650). Crosslinked products were separated on 15% SDS/PAGE and bands are stained with Coomassie brilliant blue. Lane 1, not reduced; lane 2, reduced; lanes 3–6 with ethyleneglycol bis(-succinimidylsuccinate) crosslinking concentrations of 0.1, 0.5, 2.0, and 5.0 mM. Molecular weight standards are shown. Bands corresponding to monomer, dimer, and trimer are indicated.
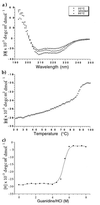
Figure 2
(a) CD spectra of pIIGp2(552–650) recorded at 20°C and 95°C, and after one cycle of heating to 95°C and cooling down to 20°C (refolded). (b) Thermal denaturation curve of pIIGp2(552–650) recorded at 222 nm with a scan rate of 1°C per min. (c) Denaturation of pIIGp2(552–650) in guanidine hydrochloride. The CD signal was monitored at 222 nm.
Figure 3
Electron micrographs of pIIGp2(552–650). Diagrammatic representations of the molecules are shown. The average length of the rods is 13 nm.
Figure 4
Electron micrographs of influenza virus TBHA2 (proteolytic fragment of low pH-treated viral HA, HA2 residues 38–175; (20); influenza virus E.TBHA2 (_E. coli_-expressed HA2 residues 38–175; (21); HIV-1 Gp41 (residues 21–166, expressed in insect cells) (22); HIV-1 pIIGpPT (proteolytic fragment derived from E. coli; pIIGCN4 and gp41 residues 30–90 and 107- 167/+12; (23); and Ebola pIIGp2(552–650). Diagrammatic representations of the molecules are shown. Micrographs showing fields of particles are found in refs. –.
Similar articles
- Crystal structure of the Ebola virus membrane fusion subunit, GP2, from the envelope glycoprotein ectodomain.
Weissenhorn W, Carfí A, Lee KH, Skehel JJ, Wiley DC. Weissenhorn W, et al. Mol Cell. 1998 Nov;2(5):605-16. doi: 10.1016/s1097-2765(00)80159-8. Mol Cell. 1998. PMID: 9844633 - Core structure of the envelope glycoprotein GP2 from Ebola virus at 1.9-A resolution.
Malashkevich VN, Schneider BJ, McNally ML, Milhollen MA, Pang JX, Kim PS. Malashkevich VN, et al. Proc Natl Acad Sci U S A. 1999 Mar 16;96(6):2662-7. doi: 10.1073/pnas.96.6.2662. Proc Natl Acad Sci U S A. 1999. PMID: 10077567 Free PMC article. - Designed protein mimics of the Ebola virus glycoprotein GP2 α-helical bundle: stability and pH effects.
Harrison JS, Higgins CD, Chandran K, Lai JR. Harrison JS, et al. Protein Sci. 2011 Sep;20(9):1587-96. doi: 10.1002/pro.688. Epub 2011 Aug 3. Protein Sci. 2011. PMID: 21739501 Free PMC article. - Structural basis for membrane fusion by enveloped viruses.
Weissenhorn W, Dessen A, Calder LJ, Harrison SC, Skehel JJ, Wiley DC. Weissenhorn W, et al. Mol Membr Biol. 1999 Jan-Mar;16(1):3-9. doi: 10.1080/096876899294706. Mol Membr Biol. 1999. PMID: 10332732 Review. - Maturation of HIV envelope glycoprotein precursors by cellular endoproteases.
Moulard M, Decroly E. Moulard M, et al. Biochim Biophys Acta. 2000 Nov 10;1469(3):121-32. doi: 10.1016/s0304-4157(00)00014-9. Biochim Biophys Acta. 2000. PMID: 11063880 Review.
Cited by
- Stoichiometry of envelope glycoprotein trimers in the entry of human immunodeficiency virus type 1.
Yang X, Kurteva S, Ren X, Lee S, Sodroski J. Yang X, et al. J Virol. 2005 Oct;79(19):12132-47. doi: 10.1128/JVI.79.19.12132-12147.2005. J Virol. 2005. PMID: 16160141 Free PMC article. - Novel ring structure in the gp41 trimer of human immunodeficiency virus type 1 that modulates sensitivity and resistance to broadly neutralizing antibodies.
O'Rourke SM, Schweighardt B, Scott WG, Wrin T, Fonseca DP, Sinangil F, Berman PW. O'Rourke SM, et al. J Virol. 2009 Aug;83(15):7728-38. doi: 10.1128/JVI.00688-09. Epub 2009 May 27. J Virol. 2009. PMID: 19474108 Free PMC article. - Direct Visualization of Ebola Virus Fusion Triggering in the Endocytic Pathway.
Spence JS, Krause TB, Mittler E, Jangra RK, Chandran K. Spence JS, et al. mBio. 2016 Feb 9;7(1):e01857-15. doi: 10.1128/mBio.01857-15. mBio. 2016. PMID: 26861015 Free PMC article. - LearnCoil-VMF: computational evidence for coiled-coil-like motifs in many viral membrane-fusion proteins.
Singh M, Berger B, Kim PS. Singh M, et al. J Mol Biol. 1999 Jul 30;290(5):1031-41. doi: 10.1006/jmbi.1999.2796. J Mol Biol. 1999. PMID: 10438601 Free PMC article. - Quaternary structure of coronavirus spikes in complex with carcinoembryonic antigen-related cell adhesion molecule cellular receptors.
Lewicki DN, Gallagher TM. Lewicki DN, et al. J Biol Chem. 2002 May 31;277(22):19727-34. doi: 10.1074/jbc.M201837200. Epub 2002 Mar 23. J Biol Chem. 2002. PMID: 11912215 Free PMC article.
References
- Bowen E T, Lloyd G, Harris W J, Platt G S, Baskerville A, Vella E E. Lancet. 1977;1:571–573. - PubMed
- Centers for Disease Control and Prevention. Morbid Mortal Week Rep. 1995;44:381–384.
- Feldmann H, Muhlberger E, Randolf A, Will C, Kiley M P, Sanchez A, Klenk H D. Virus Res. 1992;24:1–19. - PubMed
- Sanchez A, Kiley M P, Holloway B P, Auperin D D. Virus Res. 1993;29:215–240. - PubMed
- Volchkov, V. E., Volchkova, V. A., Slenczka, W., Klenk, H.-D. & Feldmann, H. (1998) Virology, in press. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous