FtsI and FtsW are localized to the septum in Escherichia coli - PubMed (original) (raw)
FtsI and FtsW are localized to the septum in Escherichia coli
L Wang et al. J Bacteriol. 1998 Jun.
Abstract
The localization of FtsI (PBP3), a penicillin-binding protein specifically required for cell division in Escherichia coli, was investigated by immunofluorescence microscopy and found to localize to the septum. The localization of FtsI was not observed in ftsZ or ftsA mutants, indicating that it was dependent on the prior localization of these proteins. Addition of furazlocillin, a specific inhibitor of FtsI, prevented localization of FtsI even though FtsZ and FtsA localization occurred. Interestingly, the localization of FtsN was also prevented by furazlocillin. FtsZ displayed limited localization in furazlocillin-treated cells, whereas it was efficiently localized in FtsI-depleted cells. FtsW, another essential cell division protein, was also localized to the septum.
Figures
FIG. 1
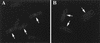
Localization of FtsI in exponentially growing cells. (A) An exponential culture of MC4100 was processed for immunofluorescence microscopy and stained with antibodies to FtsI. The arrows indicate cells that have a clear band of fluorescence at midcell. (B) Cells were stained as for panel A except that the blocking peptide was added. The arrows indicate cells with constrictions that lack the bright band of fluorescence seen in panel A.
FIG. 2
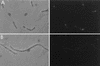
Use of an FtsI depletion strain to examine the specificity of the FtsI peptide antibody. An exponential culture of JE7947(pHR295) growing with IPTG was centrifuged, washed, and resuspended in medium lacking IPTG. Samples were taken 0 (A) and 180 (B) min later and processed for immunofluorescence microscopy using the FtsI peptide-specific antibodies. Left, phase-contrast photomicrograph; right, fluorescence photomicrograph.
FIG. 3
Western analysis of FtsI levels in strains used for FtsI localization. Cell lysates of the various strains used in this study were analyzed by Western blotting with the anti-FtsI peptide-specific antibodies. JE7947(pHR295) was sampled at 0, 60, and 180 min after removal of IPTG. MC4100 was sampled in the absence and 60 min after the addition of furazlocillin. Samples of the temperature-sensitive mutants MCZ84 and MCA12 were taken from cultures growing at 30°C and 30 min after a shift to 42°C. Samples were adjusted so that an equivalent amount of OD600 material was added in each lane.
FIG. 4
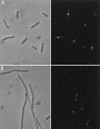
FtsI is not localized in ftsA and ftsZ filaments. Exponential cultures of MCZ84 [ftsZ84(Ts)] (A) and MCA12 [ftsA12(Ts)] (B) growing at 30°C were shifted to 42°C for 30 min. Samples were taken and immunostained for FtsI. Left, phase-contrast micrographs; right, fluorescence photomicrographs. Samples were also analyzed by Western blotting to determine the level of FtsI (Fig. 3).
FIG. 5
FtsZ localization in FtsI-depleted filaments. An exponential culture of JE7947(pHR295) growing with IPTG was centrifuged, washed, and resuspended in medium lacking IPTG. Cells were processed for immunofluorescence microscopy at 0 (A) and 180 (B) min after IPTG removal. Left, phase-contrast photomicrographs; right, fluorescence photomicrographs.
FIG. 6
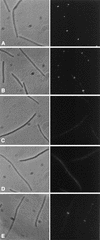
Effect of furazlocillin on the localization of Fts proteins. Furazlocillin (0.25 μg/ml) was added to an exponential culture of MC4100. Samples were taken before and 60 min after the addition. The samples were processed for immunostaining or for Western blotting (Fig. 3). The localization results are presented as a pair of photographs, phase contrast (left) and immunofluorescence (right). (A) Stained for FtsZ; (B) stained for FtsA; (C) stained for FtsI; (D) stained for FtsN; (E) stained for FtsK.
FIG. 7
Model for the assembly of proteins at the septum. In this model, FtsZ polymerizes into the Z ring at the septum. This is accompanied by ZipA and FtsA, both of which interact with FtsZ. Although FtsA has been shown to follow FtsZ, this has not been demonstrated for ZipA and it is possible that ZipA precedes FtsZ. Following these three proteins, FtsN, FtsI, and FtsK are localized to the septum. FtsW is also localized; however, it is not clear at what point it assembles, although genetic studies suggest that it acts early. FtsQ is required for FtsN assembly, but it is not known if it is assembled at the septum. Since it is not clear at which point FtsW and ZipA are recruited to the septum, they are listed below the diagram.
FIG. 8
Localization of FtsW to the septum. An exponential culture of MC4100 was processed for immunofluorescence microscopy and stained with antibodies to FtsW. Left, phase-contrast photomicrograph; right, immunofluorescence photomicrograph. Cells are observed with a band of fluorescence at midcell. In a few cells, a spot of fluorescence is observed at the cell pole; in others (<1%), the staining is not a clear pattern.
FIG. 9
Use of an FtsW depletion strain to examine the specificity of the FtsW peptide antibody. DBWC2 growing in the presence of arabinose was centrifuged, washed, and resuspended in medium containing glucose. Samples were taken at 0 (A) and 180 (B) min after resuspension and stained with the FtsW peptide-specific antibodies for fluorescence microscopy. Left, phase-contrast micrographs; right, fluorescence micrographs.
FIG. 10
Western analysis of an FtsW depletion strain. DBWC2 growing in the presence of arabinose was centrifuged, washed, and resuspended in medium containing glucose. Samples were taken at 0, 60, and 180 min after resuspension and analyzed by Western blotting using FtsW peptide-specific antibodies.
Similar articles
- FtsQ, FtsL and FtsI require FtsK, but not FtsN, for co-localization with FtsZ during Escherichia coli cell division.
Chen JC, Beckwith J. Chen JC, et al. Mol Microbiol. 2001 Oct;42(2):395-413. doi: 10.1046/j.1365-2958.2001.02640.x. Mol Microbiol. 2001. PMID: 11703663 - The Escherichia coli cell division protein FtsW is required to recruit its cognate transpeptidase, FtsI (PBP3), to the division site.
Mercer KL, Weiss DS. Mercer KL, et al. J Bacteriol. 2002 Feb;184(4):904-12. doi: 10.1128/jb.184.4.904-912.2002. J Bacteriol. 2002. PMID: 11807049 Free PMC article. - Inactivation of FtsI inhibits constriction of the FtsZ cytokinetic ring and delays the assembly of FtsZ rings at potential division sites.
Pogliano J, Pogliano K, Weiss DS, Losick R, Beckwith J. Pogliano J, et al. Proc Natl Acad Sci U S A. 1997 Jan 21;94(2):559-64. doi: 10.1073/pnas.94.2.559. Proc Natl Acad Sci U S A. 1997. PMID: 9012823 Free PMC article. - The structure and function of Escherichia coli penicillin-binding protein 3.
Nguyen-Distèche M, Fraipont C, Buddelmeijer N, Nanninga N. Nguyen-Distèche M, et al. Cell Mol Life Sci. 1998 Apr;54(4):309-16. doi: 10.1007/s000180050157. Cell Mol Life Sci. 1998. PMID: 9614966 Free PMC article. Review. - Cytokinesis in bacteria.
Errington J, Daniel RA, Scheffers DJ. Errington J, et al. Microbiol Mol Biol Rev. 2003 Mar;67(1):52-65, table of contents. doi: 10.1128/MMBR.67.1.52-65.2003. Microbiol Mol Biol Rev. 2003. PMID: 12626683 Free PMC article. Review.
Cited by
- Functional taxonomy of bacterial hyperstructures.
Norris V, den Blaauwen T, Cabin-Flaman A, Doi RH, Harshey R, Janniere L, Jimenez-Sanchez A, Jin DJ, Levin PA, Mileykovskaya E, Minsky A, Saier M Jr, Skarstad K. Norris V, et al. Microbiol Mol Biol Rev. 2007 Mar;71(1):230-53. doi: 10.1128/MMBR.00035-06. Microbiol Mol Biol Rev. 2007. PMID: 17347523 Free PMC article. Review. - Murein (peptidoglycan) binding property of the essential cell division protein FtsN from Escherichia coli.
Ursinus A, van den Ent F, Brechtel S, de Pedro M, Höltje JV, Löwe J, Vollmer W. Ursinus A, et al. J Bacteriol. 2004 Oct;186(20):6728-37. doi: 10.1128/JB.186.20.6728-6737.2004. J Bacteriol. 2004. PMID: 15466024 Free PMC article. - Cell cycle dependent coordination of surface layer biogenesis in Caulobacter crescentus.
Herdman M, Isbilir B, von Kügelgen A, Schulze U, Wainman A, Bharat TAM. Herdman M, et al. Nat Commun. 2024 Apr 18;15(1):3355. doi: 10.1038/s41467-024-47529-5. Nat Commun. 2024. PMID: 38637514 Free PMC article. - The FtsLB subcomplex of the bacterial divisome is a tetramer with an uninterrupted FtsL helix linking the transmembrane and periplasmic regions.
Condon SGF, Mahbuba DA, Armstrong CR, Diaz-Vazquez G, Craven SJ, LaPointe LM, Khadria AS, Chadda R, Crooks JA, Rangarajan N, Weibel DB, Hoskins AA, Robertson JL, Cui Q, Senes A. Condon SGF, et al. J Biol Chem. 2018 Feb 2;293(5):1623-1641. doi: 10.1074/jbc.RA117.000426. Epub 2017 Dec 12. J Biol Chem. 2018. PMID: 29233891 Free PMC article. - Bacterial Cell Size: Multifactorial and Multifaceted.
Westfall CS, Levin PA. Westfall CS, et al. Annu Rev Microbiol. 2017 Sep 8;71:499-517. doi: 10.1146/annurev-micro-090816-093803. Annu Rev Microbiol. 2017. PMID: 28886685 Free PMC article. Review.
References
- Addinall S G, Cao C, Lutkenhaus J. Temperature shift experiments with an ftsZ84(Ts) strain reveal rapid dynamics of FtsZ localization and indicate that the Z ring is required throughout septation and cannot reoccupy division sites once constriction has initiated. J Bacteriol. 1997;179:4277–4284. - PMC - PubMed
- Addinall S G, Cao C, Lutkenhaus J. FtsN, a late recruit to the septum in E. coli. Mol Microbiol. 1997;25:303–310. - PubMed
- Addinall S G, Lutkenhaus J. FtsZ spirals and arcs determine the shape of the invaginating septa in some mutants of Escherichia coli. Mol Microbiol. 1996;22:231–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases