Nonnative proteins induce expression of the Bacillus subtilis CIRCE regulon - PubMed (original) (raw)
Nonnative proteins induce expression of the Bacillus subtilis CIRCE regulon
A Mogk et al. J Bacteriol. 1998 Jun.
Free PMC article
Abstract
The chaperone-encoding groESL and dnaK operons constitute the CIRCE regulon of Bacillus subtilis. Both operons are under negative control of the repressor protein HrcA, which interacts with the CIRCE operator and whose activity is modulated by the GroESL chaperone machine. In this report, we demonstrate that induction of the CIRCE regulon can also be accomplished by ethanol stress and puromycin. Introduction of the hrcA gene and a transcriptional fusion under the control of the CIRCE operator into Escherichia coli allowed induction of this fusion by heat shock, ethanol stress, and overproduction of GroESL substrates. The expression level of this hrcA-bgaB fusion inversely correlated with the amount of GroE machinery present in the cells. Therefore, all inducing conditions seem to lead to induction via titration of the GroE chaperonins by the increased level of nonnative proteins formed. Puromycin treatment failed to induce the sigmaB-dependent general stress regulon, indicating that nonnative proteins in general do not trigger this response. Reconstitution of HrcA-dependent heat shock regulation of B. subtilis in E. coli and complementation of E. coli groESL mutants by B. subtilis groESL indicate that the GroE chaperonin systems of the two bacterial species are functionally exchangeable.
Figures
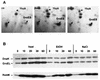
FIG. 1
Influence of heat shock, ethanol, and salt stress on levels of GroEL, DnaK, and RsbW. B. subtilis PY22 was grown in LB, and stresses were imposed during exponential growth by transferring the culture from 37 to 48°C or by adding ethanol (EtOH) or NaCl to a final concentration of 4% (vol/vol or wt/vol, respectively). (A) Sections of Coomassie blue R-350-stained two-dimensional protein gels prepared from crude extracts of growing cells (co) or cells harvested 90 min after imposition of stress. Besides GroES, the ςB-dependent stress protein YkzA and a vegetative protein are labeled. (B) Equal amounts of crude protein extracts (50 μg per lane) prepared from bacteria harvested at the time points (minutes) indicated were separated by SDS-PAGE. After transfer to a nitrocellulose membrane, levels of GroEL, DnaK, and RsbW were determined with specific antibodies raised against the corresponding proteins as described previously (3, 29, 32).
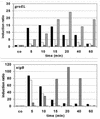
FIG. 2
Levels of groEL and sigB mRNA in_B. subtilis_ after challenge with heat, ethanol, or salt stress and puromycin. Serial dilutions of total RNA prepared from_B. subtilis_ PY22 before (co) and at 5, 10, 15, 20, 40, and 60 min after exposure to stress were bound to a positively charged nylon membrane and hybridized with the digoxigenin-labeled antisense RNA probes specific for groEL and sigB (18, 39). The hybridization signals were quantified with a fluorimager. The mRNA level in the control prior to stress was set to 1, and the induction ratios are shown. Stresses were triggered as described in Materials and Methods. ▪, 50°C; □, 4% ethanol; ▧, 4% NaCl; ░⃞, 20 μg of puromycin per ml.
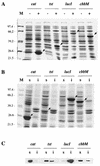
FIG. 3
Overproduction and localization of chloramphenicol acetyltransferase and GroEL substrates in E. coli. E. coli DH10B transformed with pREP9, pREP9-tst, pREP9-lucI, or pREP9-cbbM, permitting overproduction of chloramphenicol acetyltransferase (cat), rhodanese (tst), luciferase (lucI), or RubisCO (cbbM), respectively, were grown to mid-exponential phase and induced with 1 mM IPTG. (A) Whole-cell fractions corresponding to identical amounts of cell culture were collected before (−) or 2 h after (+) the addition of IPTG and resolved by SDS-PAGE. Molecular masses (in kilodaltons) of marker proteins (M) are given; arrowheads indicate localization of the overproduced proteins. (B) Soluble (s) and insoluble (i) fractions of an extract prepared from a culture induced for 2 h with IPTG were prepared as described in Materials and Methods. Aliquots corresponding to identical amounts of cell culture were loaded onto all lanes. (C) Samples identical to those in panel B were resolved by SDS-PAGE and transferred to nitrocellulose. The membranes were probed with anti-chloramphenicol acetyltransferase, antirhodanese, antiluciferase, or anti-RubisCO antibodies and developed by a colorimetric assay.
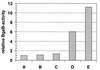
FIG. 4
Depletion of GroEL induces an _hrcA-bgaB_transcriptional fusion in E. coli. E. coli A190 carrying the two plasmids pAM103 and pBAD33-groESL(expressing the E. coli groESL genes) was grown in the presence of 0.2% arabinose in LB overnight. The cells were then washed to remove the inducer arabinose and resuspended in LB medium either in the absence or in the presence of the indicated arabinose concentrations or in the presence of 0.5% glucose as anti-inducer. Expression of the hrcA-bgaB transcriptional fusion expressed from pAM103 was assayed 4 h after the resuspension. A, 0.2% arabinose; B, 0.06% arabinose; C, 0.02% arabinose; D, no arabinose; E, 0.5% glucose.
Similar articles
- The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis.
Mogk A, Homuth G, Scholz C, Kim L, Schmid FX, Schumann W. Mogk A, et al. EMBO J. 1997 Aug 1;16(15):4579-90. doi: 10.1093/emboj/16.15.4579. EMBO J. 1997. PMID: 9303302 Free PMC article. - hrcA, the first gene of the Bacillus subtilis dnaK operon encodes a negative regulator of class I heat shock genes.
Schulz A, Schumann W. Schulz A, et al. J Bacteriol. 1996 Feb;178(4):1088-93. doi: 10.1128/jb.178.4.1088-1093.1996. J Bacteriol. 1996. PMID: 8576042 Free PMC article. - Microbial molecular chaperones.
Lund PA. Lund PA. Adv Microb Physiol. 2001;44:93-140. doi: 10.1016/s0065-2911(01)44012-4. Adv Microb Physiol. 2001. PMID: 11407116 Review. - Heat-shock and general stress response in Bacillus subtilis.
Hecker M, Schumann W, Völker U. Hecker M, et al. Mol Microbiol. 1996 Feb;19(3):417-28. doi: 10.1046/j.1365-2958.1996.396932.x. Mol Microbiol. 1996. PMID: 8830234 Review.
Cited by
- Chlamydial GroEL autoregulates its own expression through direct interactions with the HrcA repressor protein.
Wilson AC, Wu CC, Yates JR 3rd, Tan M. Wilson AC, et al. J Bacteriol. 2005 Nov;187(21):7535-42. doi: 10.1128/JB.187.21.7535-7542.2005. J Bacteriol. 2005. PMID: 16237037 Free PMC article. - Acidogenesis, solventogenesis, metabolic stress response and life cycle changes in Clostridium beijerinckii NRRL B-598 at the transcriptomic level.
Patakova P, Branska B, Sedlar K, Vasylkivska M, Jureckova K, Kolek J, Koscova P, Provaznik I. Patakova P, et al. Sci Rep. 2019 Feb 4;9(1):1371. doi: 10.1038/s41598-018-37679-0. Sci Rep. 2019. PMID: 30718562 Free PMC article. - Heat shock proteome of Agrobacterium tumefaciens: evidence for new control systems.
Rosen R, Büttner K, Becher D, Nakahigashi K, Yura T, Hecker M, Ron EZ. Rosen R, et al. J Bacteriol. 2002 Mar;184(6):1772-8. doi: 10.1128/JB.184.6.1772-1778.2002. J Bacteriol. 2002. PMID: 11872730 Free PMC article. - ClpE from Lactococcus lactis promotes repression of CtsR-dependent gene expression.
Varmanen P, Vogensen FK, Hammer K, Palva A, Ingmer H. Varmanen P, et al. J Bacteriol. 2003 Sep;185(17):5117-24. doi: 10.1128/JB.185.17.5117-5124.2003. J Bacteriol. 2003. PMID: 12923084 Free PMC article.
References
- Antelmann H, Bernhardt J, Schmid R, Mach H, Völker U, Hecker M. First steps from a two-dimensional protein index towards a response-regulation map for Bacillus subtilis. Electrophoresis. 1997;18:1451–1463. - PubMed
- Craig E A, Gross C A. Is hsp70 the cellular thermometer? Trends Biochem Sci. 1991;16:135–140. - PubMed
- Derre I, Msadek T. Presented at the 9th International Conference on Bacilli, Lausanne, Switzerland. 1997.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources