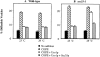Sec35p, a novel peripheral membrane protein, is required for ER to Golgi vesicle docking - PubMed (original) (raw)
Sec35p, a novel peripheral membrane protein, is required for ER to Golgi vesicle docking
S M VanRheenen et al. J Cell Biol. 1998.
Abstract
SEC35 was identified in a novel screen for temperature-sensitive mutants in the secretory pathway of the yeast Saccharomyces cerevisiae (. Genetics. 142:393-406). At the restrictive temperature, the sec35-1 strain exhibits a transport block between the ER and the Golgi apparatus and accumulates numerous vesicles. SEC35 encodes a novel cytosolic protein of 32 kD, peripherally associated with membranes. The temperature-sensitive phenotype of sec35-1 is efficiently suppressed by YPT1, which encodes the rab-like GTPase required early in the secretory pathway, or by SLY1-20, which encodes a dominant form of the ER to Golgi target -SNARE-associated protein Sly1p. Weaker suppression is evident upon overexpression of genes encoding the vesicle-SNAREs SEC22, BET1, or YKT6. The cold-sensitive lethality that results from deleting SEC35 is suppressed by YPT1 or SLY1-20. These genetic relationships suggest that Sec35p acts upstream of, or in conjunction with, Ypt1p and Sly1p as was previously found for Uso1p. Using a cell-free assay that measures distinct steps in vesicle transport from the ER to the Golgi, we find Sec35p is required for a vesicle docking stage catalyzed by Uso1p. These genetic and biochemical results suggest Sec35p acts with Uso1p to dock ER-derived vesicles to the Golgi complex.
Figures
Figure 1
Predicted amino acid sequence of Sec35p and Sec35-1p. (a) Protein sequence of Sec35p. SEC35 was sequenced as part of the Yeast Genome Sequencing project and corresponds to ORF YGR120c, GenBank/EMBL/DDBJ accession number Z72905. (b) Sequence of wild-type SEC35 from bp 613 to 628 with the transversion mutation at bp 624 (t to a) and corresponding codon change (tyrosine to ochre) present in the sec35-1 allele indicated with arrows.
Figure 2
A haploid strain containing a deletion of SEC35 exhibits a severe growth defect at 30°C and is inviable at 21°C; the cold-sensitive lethality can be rescued by the expression of SEC35, low levels of SLY1-20, or high levels of YPT1. The sec35Δ/SEC35 heterozygous deletion strain alone (a and b), or transformed with p_SEC35 (CEN)_ (c), p_YPT1 (2μm)_ (d), or p_SLY1-20 (CEN)_ (e) was sporulated and dissected. The resulting tetrads were incubated at 30°C for 7 d (a) or at 21°C for 5 d (b and c) or 7 d (d and e) on YPD plates. In c–e, only tetrads containing four viable spores are shown; tetrads with two or three viable spores were assumed to result from tetrads in which one or both of the sec35Δ haploid spores failed to receive the suppressing plasmid.
Figure 3
Suppression of the temperature-sensitive growth phenotype of the sec35-1 strain. Wild-type and sec35-1 strains containing the indicated plasmids were grown to stationary phase in synthetic media and used for a series of 10-fold dilutions. The stationary phase culture and five serial dilutions of each strain were spotted onto YPD plates and grown for 4 d at 37.5°C.
Figure 6
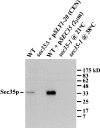
Characterization of the affinity-purified anti-Sec35p antibody. Extracts were made by glass bead lysis from logarithmically growing strains: wild-type, sec35Δ transformed with p_SLY1-20 (CEN), wild-type transformed with p_SEC35 (2μm), and sec35-1. All strains were incubated at 21°C before lysis except for the sec35-1 strain, which was maintained at 21°C or shifted to 38°C for 1 h before lysis. Extracts (0.2 OD595 per lane) were separated by SDS-12%PAGE and immunoblotted with affinity-purified anti-Sec35p antibodies. The migration of mol wt markers is shown on the right.
Figure 4
Suppression of the ER to Golgi transport defect of sec35-1 by expression of SLY1-20 or high levels of YPT1. Wild-type and sec18-1 strains, as well as the sec35-1 strain with and without the indicated plasmids, were grown at 21°C to mid-logarithmic phase and then shifted to 39°C for 45 min. Cells were labeled at 39°C for 10 min with Tran35S-label and then chased for 20 min at the same temperature. CPY was immunoprecipitated from each strain, resolved by SDS-7%PAGE, and then visualized by autoradiography. The migration of the ER precursor (p1) and mature (m) forms of CPY are indicated on the left.
Figure 5
The sec35-1 allele exhibits a synthetic interaction with ypt1-3 and uso1-1. The sec35-1 strain was mated to a strain bearing the ypt1-3 allele (a) or the uso1-1 allele (b), and the resulting diploid strains were sporulated, dissected on YPD plates, and then incubated at 30°C for 3 d. Six representative tetrads of each dissection are shown.
Figure 7
Sec35p is a peripheral membrane protein. (a) Sec35p partitions into soluble and membrane-associated pools. A wild-type strain was grown to logarithmic phase, lysed with glass beads, and then centrifuged at 1,000 g for 3 min to generate the S1 supernatant fraction. The S1 supernatant was centrifuged at 175,000 g to obtain the S175 supernatant and P175 pellet fractions. (b) The membrane-associated portion of Sec35p fractionates similarly to the Golgi protein, Sed5p. The S1 supernatant was centrifuged at 10,000 g to generate the S10 supernatant and P10 pellet fractions, and the S10 was subsequently centrifuged at 175,000 g to obtain the S175 supernatant and P175 pellet fractions. (c) Sec35p behaves as a peripheral membrane protein. The S1 supernatant was incubated with buffer 88, 1% Triton X-100, 1 M NaCl, or 100 mM Na2CO3, pH 11, centrifuged at 175,000 g, and separated into supernatant and pellet fractions. (a–c) Aliquots of each fraction were separated by SDS-12%PAGE and immunoblotted with affinity-purified anti-Sec35p (top panel), anti-Sed5p (center panel), or anti-PGK (bottom panel) antibodies. The samples loaded in each lane were derived from equivalent amounts of starting material.
Figure 8
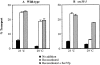
Sec35p is required for ER to Golgi transport in vitro. Semi-intact yeast cell membranes prepared from wild-type (A) or sec35-1 (B) strains were incubated at 23° or 29°C for 60 min. Reactions contained an ATP regeneration system alone (No addition, solid bars), COPII, Uso1p, and LMA1 (Reconstituted, open bars), or COPII, Uso1p, LMA1 and His6-Sec35p (Reconstituted + Sec35p, hatched bars). Outer-chain modified forms of [35S]gp-α-factor were immunoprecipitated with anti–α1,6-mannose–specific antibodies and the percent transport reflects the amount of total [35S]gp-α-factor that has acquired Golgi-specific outer-chain modifications.
Figure 9
Sec35p is required for vesicle docking. Semi-intact yeast cell membranes prepared from wild-type or sec35-1 strains were incubated at 23° or 29°C for 30 min. Reactions contained an ATP regeneration system alone (No addition, solid bars), COPII (COPII, wide hatched bars), COPII and Uso1p (COPII + Uso1p, open bars), or COPII, Uso1p, and His6-Sec35p (COPII, Uso1p + Sec35p, narrow hatched bars). Freely diffusible vesicles containing [35S]gp-α-factor were separated from semi-intact membranes by centrifugation at 18,000 g for 3 min. The percent diffusible vesicles reflects the amount of total concanavalin A precipitable [35S]gp-α-factor contained in the 18,000 g supernatant fluid.
Similar articles
- Sec34p, a protein required for vesicle tethering to the yeast Golgi apparatus, is in a complex with Sec35p.
VanRheenen SM, Cao X, Sapperstein SK, Chiang EC, Lupashin VV, Barlowe C, Waters MG. VanRheenen SM, et al. J Cell Biol. 1999 Nov 15;147(4):729-42. doi: 10.1083/jcb.147.4.729. J Cell Biol. 1999. PMID: 10562277 Free PMC article. - Assembly of the ER to Golgi SNARE complex requires Uso1p.
Sapperstein SK, Lupashin VV, Schmitt HD, Waters MG. Sapperstein SK, et al. J Cell Biol. 1996 Mar;132(5):755-67. doi: 10.1083/jcb.132.5.755. J Cell Biol. 1996. PMID: 8603910 Free PMC article. - Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins.
Cao X, Ballew N, Barlowe C. Cao X, et al. EMBO J. 1998 Apr 15;17(8):2156-65. doi: 10.1093/emboj/17.8.2156. EMBO J. 1998. PMID: 9545229 Free PMC article. - Small GTP-binding proteins and their role in transport.
Goud B, McCaffrey M. Goud B, et al. Curr Opin Cell Biol. 1991 Aug;3(4):626-33. doi: 10.1016/0955-0674(91)90033-u. Curr Opin Cell Biol. 1991. PMID: 1663370 Review. No abstract available. - Proteins involved in vesicular transport and membrane fusion.
Waters MG, Griff IC, Rothman JE. Waters MG, et al. Curr Opin Cell Biol. 1991 Aug;3(4):615-20. doi: 10.1016/0955-0674(91)90031-s. Curr Opin Cell Biol. 1991. PMID: 1772655 Review.
Cited by
- The Drosophila Cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized Golgi architecture during spermatogenesis.
Farkas RM, Giansanti MG, Gatti M, Fuller MT. Farkas RM, et al. Mol Biol Cell. 2003 Jan;14(1):190-200. doi: 10.1091/mbc.e02-06-0343. Mol Biol Cell. 2003. PMID: 12529436 Free PMC article. - A Rab requirement is not bypassed in SLY1-20 suppression.
Ballew N, Liu Y, Barlowe C. Ballew N, et al. Mol Biol Cell. 2005 Apr;16(4):1839-49. doi: 10.1091/mbc.e04-08-0725. Epub 2005 Feb 2. Mol Biol Cell. 2005. PMID: 15689495 Free PMC article. - Detailed Analysis of the Interaction of Yeast COG Complex.
Ishii M, Lupashin VV, Nakano A. Ishii M, et al. Cell Struct Funct. 2018 Jul 19;43(2):119-127. doi: 10.1247/csf.18014. Epub 2018 Jun 14. Cell Struct Funct. 2018. PMID: 29899178 Free PMC article. - SNARE chaperone Sly1 directly mediates close-range vesicle tethering.
Duan M, Plemel RL, Takenaka T, Lin A, Delgado BM, Nattermann U, Nickerson DP, Mima J, Miller EA, Merz AJ. Duan M, et al. J Cell Biol. 2024 Jun 3;223(6):e202001032. doi: 10.1083/jcb.202001032. Epub 2024 Mar 13. J Cell Biol. 2024. PMID: 38478018 Free PMC article. - A Brucella Type IV Effector Targets the COG Tethering Complex to Remodel Host Secretory Traffic and Promote Intracellular Replication.
Miller CN, Smith EP, Cundiff JA, Knodler LA, Bailey Blackburn J, Lupashin V, Celli J. Miller CN, et al. Cell Host Microbe. 2017 Sep 13;22(3):317-329.e7. doi: 10.1016/j.chom.2017.07.017. Epub 2017 Aug 24. Cell Host Microbe. 2017. PMID: 28844886 Free PMC article.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
- Baker D, Hicke L, Rexach M, Schleyer M, Schekman R. Reconstitution of SECgene product-dependent intercompartmental protein transport. Cell. 1988;54:335–344. - PubMed
- Banfield DK, Lewis MJ, Pelham HRB. A SNARE-like protein required for traffic through the Golgi complex. Nature. 1995;375:806–809. - PubMed
- Barlowe C, Schekman R. SEC12encodes a guanine-nucleotide-exchange factor essential for transport vesicle budding from the ER. Nature. 1993;365:347–349. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM48753/GM/NIGMS NIH HHS/United States
- GM52549/GM/NIGMS NIH HHS/United States
- R37 GM052549/GM/NIGMS NIH HHS/United States
- R01 GM052549/GM/NIGMS NIH HHS/United States
- GM07312/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases