A role for Cdc42 in macrophage chemotaxis - PubMed (original) (raw)
A role for Cdc42 in macrophage chemotaxis
W E Allen et al. J Cell Biol. 1998.
Abstract
Three members of the Rho family, Cdc42, Rac, and Rho are known to regulate the organization of actin-based cytoskeletal structures. In Bac1.2F5 macrophages, we have shown that Rho regulates cell contraction, whereas Rac and Cdc42 regulate the formation of lamellipodia and filopodia, respectively. We have now tested the roles of Cdc42, Rac, and Rho in colony stimulating factor-1 (CSF-1)-induced macrophage migration and chemotaxis using the Dunn chemotaxis chamber. Microinjection of constitutively activated RhoA, Rac1, or Cdc42 inhibited cell migration, presumably because the cells were unable to polarize significantly in response to CSF-1. Both Rho and Rac were required for CSF-1-induced migration, since migration speed was reduced to background levels in cells injected with C3 transferase, an inhibitor of Rho, or with the dominant-negative Rac mutant, N17Rac1. In contrast, cells injected with the dominant-negative Cdc42 mutant, N17Cdc42, were able to migrate but did not polarize in the direction of the gradient, and chemotaxis towards CSF-1 was abolished. We conclude that Rho and Rac are required for the process of cell migration, whereas Cdc42 is required for cells to respond to a gradient of CSF-1 but is not essential for cell locomotion.
Figures
Figure 1
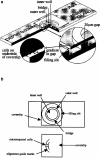
Microinjection and gradient formation in the Dunn chemotaxis chamber. (a) The Dunn chamber is a modified Helber counting chamber slide. Cells are cultured on coverslips that are then inverted onto the slide. Cells that rest over the annular bridge of the chamber can be observed under phase-contrast optics and their migration tracks are recorded automatically by time-lapse frame grabbing. (b) To measure microinjected cells, guidemarks are drawn on the coverslip to mark the limits of the Dunn chamber annular bridge and a small quadrant demarcated within a section of the alignment marks. All the cells within this quadrant are microinjected before mounting the coverslip on the chamber. Measurements of cell migration are taken for all the cells within the quadrant and a number of uninjected cells lying outside the quadrant. The latter measurements serve as internal controls.
Figure 2
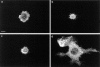
Cdc42 regulates CSF-1–induced cell polarization. Actin filament localization is shown in Bac1 cells that were starved of CSF-1 for 24 h, and either unstimulated (a), exposed to a concentration gradient of CSF-1 for 2 h (b), or microinjected with V12A35Rac1 (c), V12Cdc42 (d), or N17Cdc42 (e) followed by exposure for 2 h to a CSF-1 concentration gradient. Bar, 10 μm.
Figure 4
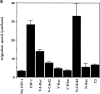
Effects of Rho, Rac, and Cdc42 proteins on the migration speed of macrophages. Bac1 macrophages were starved of CSF-1 for 24 h, microinjected with the indicated proteins, and then exposed to a gradient of CSF-1 for 3 h. The rate of cell locomotion is calculated for each cell at consecutive 4-min intervals for 3 h (a), with the mean and 95% confidence limit calculated using a Mathematica notebook. The migration tracks of 10 randomly chosen cells are plotted for V12A35Rac1-injected control cells (b), and N17Cdc42-injected cells (c) onto vector diagrams showing the final positions of all tracked cells. It is evident that N17Cdc42 does not disturb the linearity of cell migration observed in the control cells.
Figure 4
Effects of Rho, Rac, and Cdc42 proteins on the migration speed of macrophages. Bac1 macrophages were starved of CSF-1 for 24 h, microinjected with the indicated proteins, and then exposed to a gradient of CSF-1 for 3 h. The rate of cell locomotion is calculated for each cell at consecutive 4-min intervals for 3 h (a), with the mean and 95% confidence limit calculated using a Mathematica notebook. The migration tracks of 10 randomly chosen cells are plotted for V12A35Rac1-injected control cells (b), and N17Cdc42-injected cells (c) onto vector diagrams showing the final positions of all tracked cells. It is evident that N17Cdc42 does not disturb the linearity of cell migration observed in the control cells.
Figure 4
Effects of Rho, Rac, and Cdc42 proteins on the migration speed of macrophages. Bac1 macrophages were starved of CSF-1 for 24 h, microinjected with the indicated proteins, and then exposed to a gradient of CSF-1 for 3 h. The rate of cell locomotion is calculated for each cell at consecutive 4-min intervals for 3 h (a), with the mean and 95% confidence limit calculated using a Mathematica notebook. The migration tracks of 10 randomly chosen cells are plotted for V12A35Rac1-injected control cells (b), and N17Cdc42-injected cells (c) onto vector diagrams showing the final positions of all tracked cells. It is evident that N17Cdc42 does not disturb the linearity of cell migration observed in the control cells.
Figure 3
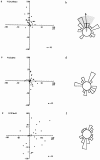
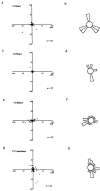
Cdc42 is required for CSF-1–induced chemotaxis but not migration. Bac1 macrophages were starved of CSF-1 for 24 h, microinjected with V12A35Rac1 (a and b), V12Cdc42 (c and d) or N17Cdc42 (e and f) and then exposed to a concentration gradient of CSF-1 for 3 h in a Dunn chamber. The migration tracks for the indicated number of cells were plotted as described in Materials and Methods. The final positions of these cells are indicated, taking the starting point for each cell as the intersection between X and Y axes, and the source of CSF-1 at the top (a, c, and e). Circular histograms (b, d, and f) show the proportion of cells with a direction of migration lying within each 20° interval, again with the source of CSF-1 at the top. Cells that migrated <10 μm are excluded from this analysis. (b) Arrow, mean direction of cell migration; grey segment; 95% confidence interval. In V12Cdc42- and N17Cdc42-injected cells (d and f), no significant directionality of migration was observed.
Figure 5
Effects of Rac1 and RhoA proteins on CSF-1-induced cell polarization. Bac1 macrophages were starved of CSF-1 for 24 h, microinjected with V12Rac1 (a), N17Rac1 (b), V14RhoA (c), or C3 transferase (d) and then exposed to a gradient of CSF-1 for 2 h in a Dunn chamber. Cells were fixed and stained to reveal actin filaments. Bar, 10 μm.
Figure 6
Rac and Rho are required for cell migration. Bac1 macrophages were starved of CSF-1 for 24 h, microinjected with V12Rac1 (a and b), N17Rac1 (c and d), V14RhoA (e and f), or C3 transferase (g and h) and then exposed to a gradient of CSF-1 for 3 h. The migration tracks for the indicated number of cells were plotted and the final positions of these cells are indicated with the starting point for each cell at the intersection between X and Y axes, with the source of CSF-1 at the top (a, c, e, and g). Circular histograms (b, d, f, and h) show the proportion of cells migrating in each direction, again with the source of CSF-1 at the top. A Rayleigh test on these data showed that there was no significant directionality of migration in any case.
Figure 7
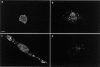
Localization of the CSF-1 receptor in migrating cells. The localization of CSF-1R (c-fms) is shown in CSF-1–starved Bac1 macrophages (a), in cells stimulated with CSF-1 for 2 min (b), in cells exposed to a concentration gradient of CSF-1 for 2 h (c), or in cells microinjected with N17Cdc42 and then exposed to a CSF-1 gradient for 2 h (d). Cells were starved of CSF-1 for 24 h before stimulation and then fixed and CSF-1R–localized with a rat anti–CSF-1R antibody followed by FITC-conjugated goat anti–rat IgG. Bar, 10 μm.
Similar articles
- Cdc42 and Rac family GTPases regulate mode and speed but not direction of primary fibroblast migration during platelet-derived growth factor-dependent chemotaxis.
Monypenny J, Zicha D, Higashida C, Oceguera-Yanez F, Narumiya S, Watanabe N. Monypenny J, et al. Mol Cell Biol. 2009 May;29(10):2730-47. doi: 10.1128/MCB.01285-08. Epub 2009 Mar 9. Mol Cell Biol. 2009. PMID: 19273601 Free PMC article. - Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages.
Allen WE, Jones GE, Pollard JW, Ridley AJ. Allen WE, et al. J Cell Sci. 1997 Mar;110 ( Pt 6):707-20. doi: 10.1242/jcs.110.6.707. J Cell Sci. 1997. PMID: 9099945 - The Rho GTPases in macrophage motility and chemotaxis.
Jones GE, Allen WE, Ridley AJ. Jones GE, et al. Cell Adhes Commun. 1998;6(2-3):237-45. doi: 10.3109/15419069809004479. Cell Adhes Commun. 1998. PMID: 9823474 Review. - Rho family proteins and cell migration.
Ridley AJ, Allen WE, Peppelenbosch M, Jones GE. Ridley AJ, et al. Biochem Soc Symp. 1999;65:111-23. Biochem Soc Symp. 1999. PMID: 10320936 Review. - Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells.
Wójciak-Stothard B, Entwistle A, Garg R, Ridley AJ. Wójciak-Stothard B, et al. J Cell Physiol. 1998 Jul;176(1):150-65. doi: 10.1002/(SICI)1097-4652(199807)176:1<150::AID-JCP17>3.0.CO;2-B. J Cell Physiol. 1998. PMID: 9618155
Cited by
- Patterning of the cell cortex by Rho GTPases.
Bement WM, Goryachev AB, Miller AL, von Dassow G. Bement WM, et al. Nat Rev Mol Cell Biol. 2024 Apr;25(4):290-308. doi: 10.1038/s41580-023-00682-z. Epub 2024 Jan 3. Nat Rev Mol Cell Biol. 2024. PMID: 38172611 Review. - Therapeutic IMC-C225 antibody inhibits breast cancer cell invasiveness via Vav2-dependent activation of RhoA GTPase.
Molli PR, Adam L, Kumar R. Molli PR, et al. Clin Cancer Res. 2008 Oct 1;14(19):6161-70. doi: 10.1158/1078-0432.CCR-07-5288. Clin Cancer Res. 2008. PMID: 18829495 Free PMC article. - Cdc42 and Rac family GTPases regulate mode and speed but not direction of primary fibroblast migration during platelet-derived growth factor-dependent chemotaxis.
Monypenny J, Zicha D, Higashida C, Oceguera-Yanez F, Narumiya S, Watanabe N. Monypenny J, et al. Mol Cell Biol. 2009 May;29(10):2730-47. doi: 10.1128/MCB.01285-08. Epub 2009 Mar 9. Mol Cell Biol. 2009. PMID: 19273601 Free PMC article. - Exocyst complex component 3-like 2 (EXOC3L2) associates with the exocyst complex and mediates directional migration of endothelial cells.
Barkefors I, Fuchs PF, Heldin J, Bergström T, Forsberg-Nilsson K, Kreuger J. Barkefors I, et al. J Biol Chem. 2011 Jul 8;286(27):24189-99. doi: 10.1074/jbc.M110.212209. Epub 2011 May 12. J Biol Chem. 2011. PMID: 21566143 Free PMC article. - Generation of membrane structures during phagocytosis and chemotaxis of macrophages: role and regulation of the actin cytoskeleton.
Rougerie P, Miskolci V, Cox D. Rougerie P, et al. Immunol Rev. 2013 Nov;256(1):222-39. doi: 10.1111/imr.12118. Immunol Rev. 2013. PMID: 24117824 Free PMC article. Review.
References
- Allen WE, Jones GE, Pollard JW, Ridley AJ. Rho, Rac and Cdc42 regulate actin organization and cell adhesion in macrophages. JCell Sci. 1997;110:707–720. - PubMed
- Aspenstrom P, Lindberg U, Hall A. Two GTPases, cdc42 and rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott-Aldrich syndrome. Curr Biol. 1996;6:70–75. - PubMed
- Baass PC, Di Guglielmo GM, Authier F, Posner BI, Bergeron JJM. Compartmentalized signal transduction by receptor tyrosine kinases. Trends Cell Biol. 1995;5:465–470. - PubMed
- Bevilacqua MP, Nelson RM, Mannori G, Cecconi O. Endothelial-leukocyte adhesion molecules in human disease. Annu Rev Med. 1994;45:361–378. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous