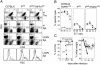Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus - PubMed (original) (raw)
Aplastic anemia rescued by exhaustion of cytokine-secreting CD8+ T cells in persistent infection with lymphocytic choriomeningitis virus
D Binder et al. J Exp Med. 1998.
Abstract
Aplastic anemia may be associated with persistent viral infections that result from failure of the immune system to control virus. To evaluate the effects on hematopoiesis exerted by sustained viral replication in the presence of activated T cells, blood values and bone marrow (BM) function were analyzed in chronic infection with lymphocytic choriomeningitis virus (LCMV) in perforin-deficient (P0/0) mice. These mice exhibit a vigorous T cell response, but are unable to eliminate the virus. Within 14 d after infection, a progressive pancytopenia developed that eventually was lethal due to agranulocytosis and thrombocytopenia correlating with an increasing loss of morphologically differentiated, pluripotent, and committed progenitors in the BM. This hematopoietic disease caused by a noncytopathic chronic virus infection was prevented by depletion of CD8+, but not of CD4+, T cells and accelerated by increasing the frequency of LCMV-specific CD8+ T cells in T cell receptor (TCR) transgenic (tg) mice. LCMV and CD8+ T cells were found only transiently in the BM of infected wild-type mice. In contrast, increased numbers of CD8+ T cells and LCMV persisted at high levels in antigen-presenting cells of infected P0/0 and P0/0 x TCR tg mice. No cognate interaction between the TCR and hematopoietic progenitors presenting either LCMV-derived or self-antigens on the major histocompatibility complex was found, but damage to hematopoiesis was due to excessive secretion and action of tumor necrosis factor (TNF)/lymphotoxin (LT)-alpha and interferon (IFN)-gamma produced by CD8+ T cells. This was studied in double-knockout mice that were genetically deficient in perforin and TNF receptor type 1. Compared with P0/0 mice, these mice had identical T cell compartments and T cell responses to LCMV, yet they survived LCMV infection and became life-long virus carriers. The numbers of hematopoietic precursors in the BM were increased compared with P0/0 mice after LCMV infection, although transient blood disease was still noticed. This residual disease activity was found to depend on IFN-gamma-producing LCMV-specific T cells and the time point of hematopoietic recovery paralleled disappearance of these virus-specific, IFN-gamma-producing CD8+ T cells. Thus, in the absence of IFN-gamma and/or TNF/LT-alpha, exhaustion of virus-specific T cells was not hampered.
Figures
Figure 1
Kinetics of peripheral blood values after infection with LCMV (109/ ml RBCs, 109/ml reticulocytes [_RC_], 109/ml thrombocytes [_TC_], 106/ml neutrophils [_NP_]). (A) C57BL/6 mice (•) or P0/0 mice (▴) were infected intravenously with LCMV (2 × 102 PFU). Blood was collected from the retroorbital plexus of individual mice at the indicated time points. CBCs were quantified in a hemocytometer and neutrophils were determined microscopically from blood smears. (B) TCR-tg P0/0 (▪) or thymectomized P0/0 mice treated with either anti-CD4 (▵) or anti-CD8 (▿) before LCMV infection. The data represent mean ± SD of four mice per group.
Figure 2
Number of stem cells in the BM after LCMV infection. (A) To determine the pluripotent hematopoietic progenitors (CFU-S), 105 syngeneic BM cells of normal or infected C57BL/6 mice (•), P0/0 (▴), or thymectomized P0/0 mice treated with either anti-CD4 (▵) or anti-CD8 (▿) donor mice were injected intravenously into lethally irradiated LCMV-immune recipient C57BL/6 mice (H-2b). CFU-S were determined 12 d later on the surface of the spleen (photograph). Each dot shows the number of colonies of an individual recipient. The horizontal lines represent the mean number of colonies per femur transferred from one individual donor mouse. The mean CFU-S of three individual donor mice per group are shown. (B) Lineage-committed precursors in C57BL/6 mice (white columns) or P0/0 (black columns) or thymectomized P0/0 mice treated with either anti-CD4 (dotted columns) or anti-CD8 (hatched columns). Results are presented as the mean number (± SD) of BFU-E, CFU-GM, or CFU-Meg for duplicate methylcellulose cultures of total BM cells per femur. Pooled data from two independent experiments with two individual mice per group are shown.
Figure 2
Number of stem cells in the BM after LCMV infection. (A) To determine the pluripotent hematopoietic progenitors (CFU-S), 105 syngeneic BM cells of normal or infected C57BL/6 mice (•), P0/0 (▴), or thymectomized P0/0 mice treated with either anti-CD4 (▵) or anti-CD8 (▿) donor mice were injected intravenously into lethally irradiated LCMV-immune recipient C57BL/6 mice (H-2b). CFU-S were determined 12 d later on the surface of the spleen (photograph). Each dot shows the number of colonies of an individual recipient. The horizontal lines represent the mean number of colonies per femur transferred from one individual donor mouse. The mean CFU-S of three individual donor mice per group are shown. (B) Lineage-committed precursors in C57BL/6 mice (white columns) or P0/0 (black columns) or thymectomized P0/0 mice treated with either anti-CD4 (dotted columns) or anti-CD8 (hatched columns). Results are presented as the mean number (± SD) of BFU-E, CFU-GM, or CFU-Meg for duplicate methylcellulose cultures of total BM cells per femur. Pooled data from two independent experiments with two individual mice per group are shown.
Figure 3
LCMV titers and infection of dendritic cells correlating with expansion of T cells in the BM. (A) LCMV titers were determined in a focus-forming assay and are given as PFU per 107 nucleated BM cells. Data points are values for three individual mice. (B) tg CD8+ T cells expressing a TCR specific for the gp peptide aa33-41 derived from LCMV presented on H-2Db were detected in the CD8+/TCR-Vα2+ compartment by FACS® analysis. Lower right quadrant, Expansion of endogenous T cells in TCR tg P0/0 (P0/0 × TCR) and CD8+ T cells of non-tg P0/0 or C57BL/6 mice. Populations represent frequencies of total nucleated BM cells and dot plots are representative of three mice. (C) Immunocytochemical staining of BM smears with an LCMV (VL4)- and CD11c (N418)-specific mAb. The respective cells expressing either LCMV or CD11c appear black.
Figure 3
LCMV titers and infection of dendritic cells correlating with expansion of T cells in the BM. (A) LCMV titers were determined in a focus-forming assay and are given as PFU per 107 nucleated BM cells. Data points are values for three individual mice. (B) tg CD8+ T cells expressing a TCR specific for the gp peptide aa33-41 derived from LCMV presented on H-2Db were detected in the CD8+/TCR-Vα2+ compartment by FACS® analysis. Lower right quadrant, Expansion of endogenous T cells in TCR tg P0/0 (P0/0 × TCR) and CD8+ T cells of non-tg P0/0 or C57BL/6 mice. Populations represent frequencies of total nucleated BM cells and dot plots are representative of three mice. (C) Immunocytochemical staining of BM smears with an LCMV (VL4)- and CD11c (N418)-specific mAb. The respective cells expressing either LCMV or CD11c appear black.
Figure 4
Comparison of different lymphocyte populations of uninfected (nl) C57BL/6, P0/0 and P0/0/TNFR10/0 mice, and consequences of LCMV infection in vivo. (A) Normal development of CD4+ and CD8+ T cell compartments in thymus (Thy) and spleen (Spl). Activation and expansion of spleen cells was compared between different mouse strains 8 d after infection with LCMV (200 PFU). Blast formation of activated T cells was demonstrated by comparing forward scatter (FSC) of CD8+ T cells from LCMV-infected (solid line) mice with uninfected (dotted line) mice. (B) LCMV-specific CTL activity measured on lymphohematopoietic target cells after a primary infection with LCMV. RMA lymphoma cells were either uninfected (open symbols) or labeled with peptide aa33-41 of LCMV-GP (•, C57BL/6; ▾, TNFR10/0 mice) presented by H-2Db. Lytic activity was measured in a 5-h51Cr– release assay; spontaneous release was <15%. (C) Overall health status after infection with LCMV as assessed by survival and loss of body weight. P0/0 mice (▴, dashed lines) were either thymectomized and depleted of CD8+ T cells (▿, dotted lines) or lacked the expression of TNFR1 (P0/0/TNFR10/0 ♦, solid lines).
Figure 5
Kinetics of CBCs and numbers of lineage-committed and pluripotent progenitors in the BM depending on expression of TNFR1 in persistent LCMV infection. (A) 109/ml RBCs, 109/ml reticulocytes [_RC_], 109/ml thrombocytes [_TC_], 106/ml neutrophils [_NP_]. Blood was collected from the retrobulbar plexus of individual TNFR10/0 (▾), P0/0 (▴), and P0/0/TNFR10/0 (♦) mice at the indicated time points (four mice per group). CBCs were quantified in a hemocytometer and neutrophils were determined microscopically from blood smears. (B) Lineage-committed precursors after LCMV infection in P0/0/TNFR10/0 (black columns) or P0/0 (white columns) mice and thymectomized P0/0/TNFR10/0 mice treated with either anti-CD4 (dotted columns) or anti-CD8 (hatched columns). Data show the mean number (± SD) of BFU-E, CFU-GM, or CFU-Meg for duplicate methylcellulose cultures of total BM cells per femur on day 14. The pluripotent hematopoietic progenitors (CFU-S) were determined as described in Materials and Methods on day 14 of infection. CFU-S in the spleens of P0/0/TNFR10/0 mice (♦) or P0/0 (▴) and thymectomized P0/0/TNFR10/0 mice treated with either anti-CD4 (□) or anti-CD8 (○) were quantified in lethally irradiated LCMV-immune recipient C57BL/6 mice (H-2b). Each dot shows the number of colonies of an individual recipient. The horizontal lines represent the mean number of colonies per femur transferred from one individual donor mouse.
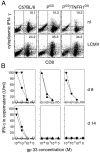
Figure 6
Production of IFN-γ by CD8+ T cells, isolated from LCMV-infected C57BL/6 and mutant P0/0 or P0/0/TNFR10/0 mice. (A) Cytokine-expressing T cells were identified in vivo in MACS®-enriched CD8+ spleen cells after stimulation with PMA and ionomycin in vitro. CD8+ T cells were isolated from spleens of either uninfected controls (nl) or mice infected 8 d previously with LCMV. The proportion of cells expressing intracellular IFN-γ was measured by FACS® analysis; the numbers in dot plots indicate percentages of cells expressing IFN-γ gated for CD8+ cells. Lower left quadrants, Cells were 20–25% of purified spleen cells and were mainly CD4+ T cells. Plots are representative for three mice per group. (B) Detection of IFN-γ–secreting virus-specific T cells at 8 and 14 d after LCMV infection in parental C57BL/6 (•) and TNFR10/0 (▾) or mutant P0/0 (▴) and P0/0/TNFR10/0 (♦) mice. Corresponding uninfected control mice of the same genotype were included in the same experimental settings (open symbols). MACS®-enriched CD8+ spleen cells of infected wt and mutant mice were restimulated in vitro with irradiated syngeneic APCs coated in threefold serial dilutions with gp33 in the presence of IL-2. After 48 h, IFN-γ was measured in an ELISA; numbers on ordinates represent IFN-γ concentrations in supernatant (U/ml).
Figure 7
Influence on RBC and neutrophil formation by neutralizing IFN-γ during chronic LCMV infection in P0/0 and P0/0/ TNFR10/0 mice. From days 5 to 15 after LCMV infection, mice were injected daily with sheep anti–IFN-γ (anti-IFN-γ) or sheep normal serum (NS) intraperitoneally. (A) Hemocytogram showing leukocyte counts in P0/0 mice treated with normal sheep serum on day 14 after LCMV infection (plot representative for four mice/treatment group). Neutrophils are identified by their positive staining for peroxidase; numbers in plots delineate neutrophils (×106/ml) determined microscopically from blood smears. (B) Circulating reticulocytes quantitated flow cytometrically by unspecific RNA staining with auramine-O. The y-axis shows forward scatter (FSC) and the x-axis shows forward fluorescence. Cells with a high fluorescence intensity represent young reticulocytes containing high amounts of RNA and are separated from the area of mature RBCs by a vertical line. The horizontal curve separates platelets from RBCs and reticulocytes. Numbers in plots are absolute numbers of reticulocytes in the blood (×106/ml). (C and D) Myeloid and erythroid precursors in the BM of mice treated with sheep normal serum (open columns) or sheep anti–IFN-γ (black columns) 14 d after LCMV infection. Values show the mean number (± SD) for duplicate methylcellulose cultures of total BM cells per femur. Pooled data from three individual mice per group are shown.
Similar articles
- Virus-induced transient bone marrow aplasia: major role of interferon-alpha/beta during acute infection with the noncytopathic lymphocytic choriomeningitis virus.
Binder D, Fehr J, Hengartner H, Zinkernagel RM. Binder D, et al. J Exp Med. 1997 Feb 3;185(3):517-30. doi: 10.1084/jem.185.3.517. J Exp Med. 1997. PMID: 9053452 Free PMC article. - Dissection of antiviral and immune regulatory functions of tumor necrosis factor receptors in a chronic lymphocytic choriomeningitis virus infection.
Suresh M, Gao X, Fischer C, Miller NE, Tewari K. Suresh M, et al. J Virol. 2004 Apr;78(8):3906-18. doi: 10.1128/jvi.78.8.3906-3918.2004. J Virol. 2004. PMID: 15047807 Free PMC article. - Critical role for perforin-, Fas/FasL-, and TNFR1-mediated cytotoxic pathways in down-regulation of antigen-specific T cells during persistent viral infection.
Zhou S, Ou R, Huang L, Moskophidis D. Zhou S, et al. J Virol. 2002 Jan;76(2):829-40. doi: 10.1128/jvi.76.2.829-840.2002. J Virol. 2002. PMID: 11752172 Free PMC article. - Chronic LCMV Infection Is Fortified with Versatile Tactics to Suppress Host T Cell Immunity and Establish Viral Persistence.
Studstill CJ, Hahm B. Studstill CJ, et al. Viruses. 2021 Sep 29;13(10):1951. doi: 10.3390/v13101951. Viruses. 2021. PMID: 34696381 Free PMC article. Review. - Complexities of Type I Interferon Biology: Lessons from LCMV.
Suprunenko T, Hofer MJ. Suprunenko T, et al. Viruses. 2019 Feb 20;11(2):172. doi: 10.3390/v11020172. Viruses. 2019. PMID: 30791575 Free PMC article. Review.
Cited by
- Murine Models of Familial Cytokine Storm Syndromes.
Volkmer B, Marchetti T, Aichele P, Schmid JP. Volkmer B, et al. Adv Exp Med Biol. 2024;1448:481-496. doi: 10.1007/978-3-031-59815-9_33. Adv Exp Med Biol. 2024. PMID: 39117835 Review. - CD8+ T Cell Biology in Cytokine Storm Syndromes.
Sekine T, Galgano D, Casoni GP, Meeths M, Cron RQ, Bryceson YT. Sekine T, et al. Adv Exp Med Biol. 2024;1448:129-144. doi: 10.1007/978-3-031-59815-9_10. Adv Exp Med Biol. 2024. PMID: 39117812 Review. - Differential effects of itacitinib, fedratinib, and ruxolitinib in mouse models of hemophagocytic lymphohistiocytosis.
Keenan C, Albeituni S, Oak N, Stroh A, Tillman HS, Wang Y, Freeman BB 3rd, Alemán-Arteaga S, Meyer LK, Woods R, Verbist KC, Zhou Y, Cheng C, Nichols KE. Keenan C, et al. Blood. 2024 Jun 6;143(23):2386-2400. doi: 10.1182/blood.2023021046. Blood. 2024. PMID: 38446698 - Depletion of Bone Marrow Hematopoietic Cells in Ebolavirus-Infected Rhesus Macaques: A Possible Cause of Hematologic Abnormalities in Ebolavirus Disease.
Liu DX, Pahar B, Perry DL, Xu H, Cooper TK, Huzella LM, Hart RJ, Hischak AMW, Bernbaum J, St Claire M, Byrum R, Bennett RS, Warren T, Holbrook MR, Hensley LE, Crozier I, Schmaljohn CS. Liu DX, et al. Am J Pathol. 2023 Dec;193(12):2031-2046. doi: 10.1016/j.ajpath.2023.08.010. Epub 2023 Sep 7. Am J Pathol. 2023. PMID: 37689386 Free PMC article. - Cellular and transcriptional impacts of Janus kinase and/or IFN-gamma inhibition in a mouse model of primary hemophagocytic lymphohistiocytosis.
Albeituni S, Oak N, Tillman HS, Stroh A, Keenan C, Bloom M, Nichols KE. Albeituni S, et al. Front Immunol. 2023 Apr 27;14:1137037. doi: 10.3389/fimmu.2023.1137037. eCollection 2023. Front Immunol. 2023. PMID: 37228616 Free PMC article.
References
- Young, N.S., and B.P. Alter. 1994. Aplastic anemia: acquired and inherited. W.B. Saunders, Philadelphia.1–267.
- Iishi Y, Kosaka M, Mizuguchi T, Toyota K, Takaue Y, Kawano Y, Saito S. Suppression of hematopoiesis by activated T-cells in infectious mononucleosis associated with pancytopenia. Int J Hematol. 1991;54:65–73. - PubMed
- Shadduck RK, Winkelstein A, Zeigler Z, Lichter J, Goldstein M, Michaels M, Rabin B. Aplastic anemia following infectious mononucleosis: possible immune etiology. Exp Hematol (NY) 1979;7:264–271. - PubMed
- Apperley JF, Dowding C, Hibbin J, Buiter J, Matutes E, Sissons PJ, Gordon M, Goldman JM. The effect of cytomegalovirus on hemopoiesis: in vitro evidence for selective infection of marrow stromal cells. Exp Hematol (NY) 1989;17:38–45. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous