B-50/GAP-43-induced formation of filopodia depends on Rho-GTPase - PubMed (original) (raw)
B-50/GAP-43-induced formation of filopodia depends on Rho-GTPase
L H Aarts et al. Mol Biol Cell. 1998 Jun.
Free PMC article
Abstract
In the present study we show that expression of the neural PKC-substrate B-50 (growth-associated protein [GAP-43]) in Rat-1 fibroblasts induced the formation of filopodial extensions during spreading. This morphological change was accompanied by an enhanced formation of peripheral actin filaments and by accumulation of vinculin immunoreactivity in filopodial focal adhesions, colocalizing with B-50. In time lapse experiments, the B-50-induced filopodial extensions were shown to stay in close contact with the substratum and appeared remarkably stable, resulting in a delayed lamellar spreading of the fibroblasts. The morphogenetic effects of the B-50 protein were entirely dependent on the integrity of the two N-terminal cysteines involved in membrane association (C3C4), but were not significantly affected by mutations of the PKC-phosphorylation site (S41) or deletion of the C terminus (177-226). Cotransfection of B-50 with dominant negative Cdc42 or Rac did not prevent B-50-induced formation of filopodial cells, whereas this process could be completely blocked by cotransfection with dominant negative Rho or Clostridium botulinum C3-transferase. Conversely, constitutively active Rho induced a similar filopodial phenotype as B-50. We therefore propose that the induction of surface extensions by B-50 in spreading Rat-1 fibroblasts depends on Rho-guanosine triphosphatase function.
Figures
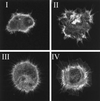
Figure 1
Morphologies of Rat-1 fibroblasts during spreading, stained for f-actin. Class I, cells with few protrusions; Class II, irregularly shaped cells; Class III, filopodial cells; and Class IV, lamellar cells.
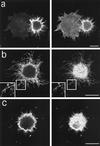
Figure 2
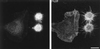
B-50–induced formation of filopodia and focal adhesions in spreading Rat-1 fibroblasts. Rat-1 fibroblasts were transfected with wild-type B-50-cDNA (in pcDNA1) using lipofectin and grown for 30 h in the absence of serum. Subsequently, cells were replated onto glass coverslips using trypsin/EDTA and fixed after 40 min. Cells were stained for B-50 (left panels) and for f-actin (a) or vinculin (b and c) (right panels) and observed by confocal laser scanning microscopy. Each figure shows a projection of several confocal planes. Inserts show enlargements of the indicated boxes. Scale bar, 10 μm.
Figure 3
Dynamics of B-50/EGFP–induced filopodial extensions in living Rat-1 fibroblasts. Rat-1 fibroblasts were transfected as described in the legend to Figure 2. Upon trypsinization, cells were replated on 24-mm glass coverslips, mounted in a microchamber, and transferred to the microscopic stage of the confocal microscope where they were kept at 37°C and 7% CO2. Upper frames show GFP-fluorescence in pB-50/GFP-transfected cells; lower frames show the corresponding phase contrast images. (a) Frames at selected timepoints after replating showing the spontaneous extrusion of long, stable, substrate-attached filopodia in a pB-50/EGFP–transfected cell. (b) Time lapse sequence of a pB-50/EGFP–transfected cell showing the transition from a filopodial to a ruffled or lamellar phenotype. Note that untransfected cells do not show a filopodial phenotype preceding lamellar spreading. Scale bar, 10 μm.
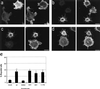
Figure 4
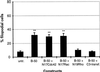
Morphologies of cells expressing B-50 mutants. Rat-1 fibroblasts were transfected with [S3G4]B-50 (a), [D41]B-50 (b), [A41]B-50 (c), or [1–176]B-50 (d) (in pcDNA1) and processed as described in the legend to Figure 2. Cells were stained for B-50 (left panels) and for f-actin (right panels) and observed by confocal laser scanning microscopy. Typical examples for cells expressing the various constructs are shown. Scale bar, 10 μm. (e) Quantitation of filopodial cell formation (type III; Figure 1). Values represent means ± SEM from three independent experiments for each construct (**, p < 0.001; *, p < 0.01).
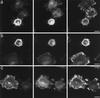
Figure 5
Effects of interference with Cdc42, Rac, or Rho function on the B-50–induced morphological changes. Rat-1 fibroblasts were cotransfected with wild-type B-50/GAP-43 and myc-epitope–tagged N17Cdc42 (a), N17Rac (b), or N19Rho (c) and processed as described in the legend to Figure 2. Cells were triple-stained for B-50 (left panels), myc-epitopes (middle panels), and f-actin (right panels) and visualized by confocal laser scanning microscopy. Scale bar, 10 μm.
Figure 6
Quantitation of filopodial cells after cotransfection with B-50 and GTPase inhibitors. Rat-1 fibroblasts were treated as described in the legend to Figure 5. Percentages of filopodial cells (type III, Figure 1) were scored by examining the f-actin staining. Values represent means ± SEM from three independent experiments for each construct (**, p < 0.001).
Figure 7
Morphological changes induced by expression of activated Rho in Rat-1 fibroblasts during spreading. Rat-1 fibroblasts were transfected with myc-epitope–tagged V14Rho and processed as described in the legend to Figure 2. Cells were stained for myc-epitopes (left panels) and FITC-coupled phalloidin (right panels) and observed by confocal laser scanning microscopy. Scale bar, 10 μm.
Figure 8
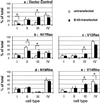
B-50–induced shifts in morphological categories of Rat-1 fibroblasts stably expressing mutant Rac- or Rho-GTPase constructs. Rat-1 fibroblasts stably expressing control plasmids (a), N17Rac (b), V12Rac (c), N19Rho (d), or V14Rho (e) were transfected with B-50 and processed as described in the legend to Figure 2. Untransfected (open bars) or wild-type B-50 transfected cells (closed bars) were classified into four morphological categories based on their f-actin staining (see Figure 1). Values represent means ± SEM from three independent experiments for each cell line (*, p < 0.05).
Similar articles
- Possible involvement of the inactivation of the Rho-Rho-kinase pathway in oncogenic Ras-induced transformation.
Izawa I, Amano M, Chihara K, Yamamoto T, Kaibuchi K. Izawa I, et al. Oncogene. 1998 Dec 3;17(22):2863-71. doi: 10.1038/sj.onc.1202213. Oncogene. 1998. PMID: 9879992 - Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42.
Threadgill R, Bobb K, Ghosh A. Threadgill R, et al. Neuron. 1997 Sep;19(3):625-34. doi: 10.1016/s0896-6273(00)80376-1. Neuron. 1997. PMID: 9331353 - Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering.
Wójciak-Stothard B, Williams L, Ridley AJ. Wójciak-Stothard B, et al. J Cell Biol. 1999 Jun 14;145(6):1293-307. doi: 10.1083/jcb.145.6.1293. J Cell Biol. 1999. PMID: 10366600 Free PMC article. - IpaC induces actin polymerization and filopodia formation during Shigella entry into epithelial cells.
Tran Van Nhieu G, Caron E, Hall A, Sansonetti PJ. Tran Van Nhieu G, et al. EMBO J. 1999 Jun 15;18(12):3249-62. doi: 10.1093/emboj/18.12.3249. EMBO J. 1999. PMID: 10369666 Free PMC article. - Ras-related GTPases and the cytoskeleton.
Hall A. Hall A. Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review.
Cited by
- A role for α-Synuclein in axon growth and its implications in corticostriatal glutamatergic plasticity in Parkinson's disease.
Schechter M, Grigoletto J, Abd-Elhadi S, Glickstein H, Friedman A, Serrano GE, Beach TG, Sharon R. Schechter M, et al. Mol Neurodegener. 2020 Mar 30;15(1):24. doi: 10.1186/s13024-020-00370-y. Mol Neurodegener. 2020. PMID: 32228705 Free PMC article. - Local accumulations of B-50/GAP-43 evoke excessive bleb formation in PC12 cells.
Aarts LH, Verkade P, Schrama LH, Oestreicher AB, Gispen WH, Schotman P. Aarts LH, et al. Mol Neurobiol. 1999 Aug;20(1):17-28. doi: 10.1007/BF02741362. Mol Neurobiol. 1999. PMID: 10595870 - Myelin and collapsin-1 induce motor neuron growth cone collapse through different pathways: inhibition of collapse by opposing mutants of rac1.
Kuhn TB, Brown MD, Wilcox CL, Raper JA, Bamburg JR. Kuhn TB, et al. J Neurosci. 1999 Mar 15;19(6):1965-75. doi: 10.1523/JNEUROSCI.19-06-01965.1999. J Neurosci. 1999. PMID: 10066250 Free PMC article. - hnRNP-Q1 represses nascent axon growth in cortical neurons by inhibiting Gap-43 mRNA translation.
Williams KR, McAninch DS, Stefanovic S, Xing L, Allen M, Li W, Feng Y, Mihailescu MR, Bassell GJ. Williams KR, et al. Mol Biol Cell. 2016 Feb 1;27(3):518-34. doi: 10.1091/mbc.E15-07-0504. Epub 2015 Dec 10. Mol Biol Cell. 2016. PMID: 26658614 Free PMC article.
References
- Aarts LHJ, Van der Linden JAM, Hage WJ, Van Rozen AJ, Oestreicher AB, Gispen WH, Schotman P. N-terminal cysteines essential for Golgi sorting of B-50 (GAP-43) in PC12 cells. Neuroreport. 1995;6:969–972. - PubMed
- Aigner L, Arber S, Kapfhammer JP, Laux T, Schneider C, Botteri F, Brenner HR, Caroni P. Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell. 1995;83:269–278. - PubMed
- Apel ED, Litchfield DW, Clark RH, Krebs EG, Storm DR. Phosphorylation of neuromodulin (GAP-43) by casein kinase II; identification of phosphorylation sites and regulation by calmodulin. J Biol Chem. 1991;16:10544–10551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous