Dopamine neurons make glutamatergic synapses in vitro - PubMed (original) (raw)
Dopamine neurons make glutamatergic synapses in vitro
D Sulzer et al. J Neurosci. 1998.
Abstract
Interactions between dopamine and glutamate play prominent roles in memory, addiction, and schizophrenia. Several lines of evidence have suggested that the ventral midbrain dopamine neurons that give rise to the major CNS dopaminergic projections may also be glutamatergic. To examine this possibility, we double immunostained ventral midbrain sections from rat and monkey for the dopamine-synthetic enzyme tyrosine hydroxylase and for glutamate; we found that most dopamine neurons immunostained for glutamate, both in rat and monkey. We then used postnatal cell culture to examine individual dopamine neurons. Again, most dopamine neurons immunostained for glutamate; they were also immunoreactive for phosphate-activated glutaminase, the major source of neurotransmitter glutamate. Inhibition of glutaminase reduced glutamate staining. In single-cell microculture, dopamine neurons gave rise to varicosities immunoreactive for both tyrosine hydroxylase and glutamate and others immunoreactive mainly for glutamate, which were found near the cell body. At the ultrastructural level, dopamine neurons formed occasional dopaminergic varicosities with symmetric synaptic specializations, but they more commonly formed nondopaminergic varicosities with asymmetric synaptic specializations. Stimulation of individual dopamine neurons evoked a fast glutamatergic autaptic EPSC that showed presynaptic inhibition caused by concomitant dopamine release. Thus, dopamine neurons may exert rapid synaptic actions via their glutamatergic synapses and slower modulatory actions via their dopaminergic synapses. Together with evidence for glutamate cotransmission in serotonergic raphe neurons and noradrenergic locus coeruleus neurons, the present results suggest that glutamatergic cotransmission may be the rule for central monoaminergic neurons.
Figures
Fig. 1.
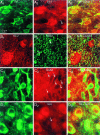
Immunostaining of DA neurons in VM sections for GLU and GABA. Coronal sections of rat and monkey VM were double-immunofluorescence-stained for the DA synthetic enzyme TH and GLU or GABA. A, In rat VM, the majority of DA neurons (A 1) were GLUergic (A 2); occasional DA neurons (A 2, arrow) were non-GLUergic. In a color merge (A 3), in which colocalization appears yellow, neuronal nuclei appear red, reflecting selective GLU staining because TH is cytoplasmic. The dense cortical GLUergic projection to the DA cell groups accounts for the strong GLUergic staining of the neuropil.B, GLUergic (B 1) neurons are not GABAergic (B 2), arguing that precursor GLU does (Figure legend continues). not account for GLU staining; this is shown as a color merge in B 3. In this field, both GLU+ neurons are GABA−(B 2, arrows). C, Furthermore, DA neurons (C 1) are never GABAergic (C 2); this is seen more clearly in the color merge (C 3). In this field, all nine DA neurons are GABA− (some are indicated by_C_ 2, arrows). D, In monkey VM, the majority of DA neurons (D 1) were GLUergic (D 2). Occasional DA neurons (D 2, arrow) were non-GLUergic. This is shown as a color merge in D3.
Fig. 2.
Immunostaining of DA neurons for GLU in vitro. To evaluate GLU staining of DA neurons, mass cultures of VTA, cerebellum, and hippocampus were immunostained for GLU and GABA.A, In vitro the majority of DA neurons in VM cultures (A 1) were GLUergic (A 2); occasional DA neurons (A 2, arrow) were non-GLUergic. In the color merge (A 3), in which colocalization appears yellow, neuronal nuclei appear_red_, reflecting selective GLU staining. A neuron that is neither TH+ nor GLU+ is seen (A 3, arrow). B, In a cerebellar culture in which granule cells, which are small and GLUergic, can be distinguished from Purkinje cells, which are large and GABAergic, only the putative large Purkinje cell stains for GABA (B 1), whereas the two granule cells do not stain (arrows). However, both the Purkinje cell as well as the granule cells appear GLUergic (B 2). This is seen more clearly in the color merge (B 3). In this experiment, all large neurons were GABA+ and GLU+ (n = 16), whereas all small neurons were only GLU+ (n = 40). This indicates that in vitro GABA neurons contain appreciable GLU, which is likely to be present as a precursor to GABA.C, Hippocampal neurons are either GLUergic (majority) or GABAergic (minority). In this culture, occasional cells stained for GABA (C 1, arrow), whereas most stained for GLU (C 2). In this experiment, 100% of GABA+ neurons were also GLU+, whereas 27% of GLU+ neurons were GABA+ (n = 38). So again, GLU staining appears to identify cells that are GLUergic as well as GABAergic cells, whereas GLU is likely present as a precursor to GABA.D, Immunostaining of precursor GLU was not so much of a confound in VTA cultures because most DA neurons were not GABAergic (D 1) and most GABA neurons were not DAergic (D 2). However, occasional DA neurons were GABAergic (D 3) (see Results for incidence).
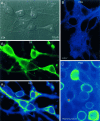
Fig. 3.
PAG immunostaining in vitro.A, In a VTA culture, six neurons are shown (A 1, numbered). All the neurons in the field except for neuron 2 are DA neurons (A 2); the level of TH staining varies in vitro as it does in vivo (Bayer and Pickel, 1990). In A 3 and in subsequent panels, staining intensity is shown on a pseudocolor scale in which warmer colors reflect more intense staining. Of the DA neurons, all except for neuron_1_ show high levels of immunoreactivity for PAG. The non-DA neuron (neuron 2) is PAG+.B, In nAcc, which is composed principally of GABAergic neurons (with a minority population of cholinergic neurons) and has no GLUergic neurons, there was no PAG staining. In this field, all six neurons are PAG−. C, In contrast, in hippocampus in which most neurons are GLUergic, most neurons stain for PAG. Here the 13 neurons in the field show varying degrees of PAG staining.
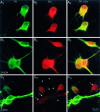
Fig. 4.
PAG inhibition reduces GLU immunostaining. In an untreated culture (A), a field is shown with two DA neurons (A 1), both of which are GLU+ (A 2,A 3). GLU staining was not diminished when cultures were pretreated with
d
-DON (5 m
m
for 20 hr), the inactive enantiomer of the irreversible PAG inhibitor (B); here three of three TH+cells (B 1) are GLU+(B 2, B 3). In contrast, with
l
-DON (5 m
m
for 20 hr), the active enantiomer, there was a significant reduction in the GLU staining of DA neurons; here two of the four DA neurons in the field (C 1) were GLU−(C 2, arrows). Although the reduction in cell body staining is not complete, there was an almost complete loss of GLU staining in DA neuron processes (C 2, arrowheads;C 3). This field also contains one TH−/GLU+ neuron (C 3, arrow), which most likely was GABAergic (see Results) and, as would be expected, showed strong GLU staining after PAG inhibition.
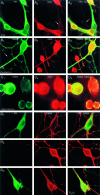
Fig. 5.
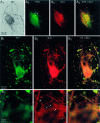
DA neurons have two overlapping sets of synaptic varicosities. A, Single DA neurons in microcultures were identified after TH staining with DAB (A 1). The culture was subsequently stained for SYN to identify presynaptic sites and then for GLU. As the DAB reaction product obscured any fluorescence immunostaining, subsequent fluorescence immunostaining was consequently restricted to TH− areas; this revealed several SYN+ presynaptic sites near the cell body (A 2; the two most prominent ones are identified by arrows). Immunostaining for GLU revealed that these sites were GLU+ (A 3,A 4). B, To examine the relationship between putative DAergic and GLUergic synaptic sites, we double (Figure legend continues). immunofluorescence stained single DA neurons in microcultures for TH and GLU. Many varicosities double stained for TH (B 1) and for GLU (B 2). Just above the cell body (the outlined region is shown 3× enlarged in each bottom panel), a cluster of smaller varicosities (bottom panel of B 2,arrows) stains primarily for GLU. Thus, DA neurons appear to have varicosities that are both DAergic and GLUergic and others that are just GLUergic.
Fig. 6.
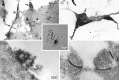
Ultrastructure of a DA neuron in single cell microculture. Sections are shown from a single DA neuron grown in microcultures and TH-stained using DAB (inset). To maximize antibody penetration, we used a relatively high detergent concentration. Although this solubilized membranes, resulting in an apparent degradation of the quality of ultrastructural preservation, it enhanced the visualization of synaptic specializations.A, In the cell body, TH staining was distributed in a patchy pattern throughout weakly stained cytoplasm. TH+ processes emerged from intensely stained regions (filled arrows); nearby, TH−processes emerged from unstained regions (open arrows).B, Within the neuropil, distinctly stained and unstained processes intermingled with each other; in some cases within a single process, a stained portion (filled arrow) was clearly distinguishable from an unstained portion (open arrow). C, Single TH-immunoreactive neurons formed morphological synapses on themselves (autapses). Those autapses were in close proximity to the cell body (as seen at the light level; Fig. 5). In this cell, a total of eight autapses with clear postsynaptic specializations were identified after serial sectioning; one autapse showed presynaptic TH staining and had symmetric synaptic membrane specializations. D, The other autapses had asymmetric synaptic specializations with no detectable immunostaining of the presynaptic elements. Two of the seven TH−boutons (data not shown) made asymmetric synaptic contacts with TH+ dendritic elements. TH+varicosities at a distance from the cell body had accumulations of synaptic vesicles but lacked presynaptic or postsynaptic densities (data not shown).
Fig. 7.
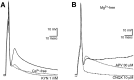
DA neurons make GLUergic EPSPs in microculture.A, Under whole-cell current clamp, a single VTA neuron in a microculture was stimulated with brief depolarizing current pulses (traces shown are averages of 6 stimulations). A large EPSP was evoked with fixed latency (solid line), which was completely blocked by local perfusion with Ca2+-free saline (dashed line), and recovered fully in physiological saline (data not shown). KYN (at a high concentration that completely blocks NMDA receptors via action at the allosteric glycine site and competitively blocks AMPA receptors with lesser potency) significantly attenuated the EPSP (gray line).B, In another cell recorded in Mg2+-free saline, a large fixed latency EPSP was evoked, which was followed by a prolonged depolarization; in some traces this went on to trigger recurrent spikes (i.e., epileptiform-like activity). This EPSP was largely blocked by CNQX; APV attenuated the later phase of the EPSP.
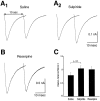
Fig. 8.
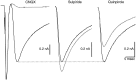
D2 modulation of GLUergic EPSC. In a neuron, subsequently shown to be TH+, a large autaptic EPSC was recorded under voltage clamp. This was almost completely blocked by CNQX (EPSC was 4% of control; traces shown are averages of 10 stimulations; traces during drug application are shown in_gray_). The reversible D2 antagonist sulpiride enhanced the EPSC (117%; shown here and in subsequent traces without the initiating action current), whereas the D2 agonist quinpirole markedly attenuated the autaptic EPSC (76%). This suggests that concomitant DA release modulates the GLUergic EPSC.
Fig. 9.
Activity-dependent DA release modulates the EPSC.A, When two stimulations were delivered separated by 10 sec, the second EPSC was significantly smaller (A 1) in contrast to the same stimulation in the presence of 1 μ
m
sulpiride (A 2). Cells were rested for a minimum of 2 min between experimental trials.B, In another cell after exposure to reserpine (1 μ
m
for 90 min) there was no significant reduction in EPSC size at stimulation 2. C, Overall in 10 such experiments, there was a significant DA-dependent inhibition at stimulation 2.
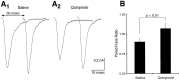
Fig. 10.
DAergic presynaptic inhibition of GLU release.A, To determine whether the locus of the D2 inhibition was presynaptic, we examined the effects of quinpirole on paired pulse responses. In saline, there was modest increase in the PPR (A 1, 112%). Quinpirole both diminished the size of the response (A 2), in this case to 71% of the response in saline, and increased the PPR (137%).B, In 10 experiments quinpirole significantly increased PPR favoring presynaptic action.
Fig. 11.
Activity-dependent presynaptic inhibition.A, A cell was stimulated with two sets of paired pulses separated by 10 sec. In saline (A 1), there was an increase in the PPR from stimulation 1 to stimulation 2, as well as a decrement in the first response at stimulation 2, whereas in sulpiride (A 2) there was neither an increase in the paired pulse ratio between stimulation 1 and stimulation 2 nor a decrease in the first response of the pair at stimulation 2.B, In 10 experiments, there was a significant difference in the PPR between saline and sulpiride at stimulation 2, consistent with activity-dependent D2-mediated presynaptic inhibition.
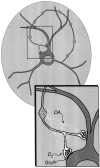
Fig. 12.
Relationship of DA neuron terminals in microculture. To illustrate the relationship between the two sets of DA neuron synapses, a schematic of a single DA neuron in microculture is shown. Regions of TH staining are shaded gray; DAergic synaptic vesicles in DAergic varicosities are shown in_white_, whereas GLUergic vesicles in GLUergic terminals are shown in black. DA neuron axons commonly arise from dendrites (Hausser et al., 1995). The area outlined by the_rectangle_ is expanded as an inset that shows DA release (small white dots) from a DAergic varicosity. This overflows to TH− GLUergic terminals, binds to D2 receptors, and mediates presynaptic inhibition. D2 autoreceptors are also present on DAergic varicosities (Rayport, 1998), which would inhibit DA release (Cragg and Greenfield, 1997). GLU receptors (GluR) are shown as forming the postsynaptic densities of asymmetric synaptic specializations.
Similar articles
- Mesoaccumbens dopamine neuron synapses reconstructed in vitro are glutamatergic.
Joyce MP, Rayport S. Joyce MP, et al. Neuroscience. 2000;99(3):445-56. doi: 10.1016/s0306-4522(00)00219-0. Neuroscience. 2000. PMID: 11029537 - Glutamate is a cotransmitter in ventral midbrain dopamine neurons.
Rayport S. Rayport S. Parkinsonism Relat Disord. 2001 Jul;7(3):261-264. doi: 10.1016/s1353-8020(00)00068-7. Parkinsonism Relat Disord. 2001. PMID: 11331197 - Synaptic innervation of midbrain dopaminergic neurons by glutamate-enriched terminals in the squirrel monkey.
Smith Y, Charara A, Parent A. Smith Y, et al. J Comp Neurol. 1996 Jan 8;364(2):231-53. doi: 10.1002/(SICI)1096-9861(19960108)364:2<231::AID-CNE4>3.0.CO;2-6. J Comp Neurol. 1996. PMID: 8788247 - Dopamine Neurons That Cotransmit Glutamate, From Synapses to Circuits to Behavior.
Eskenazi D, Malave L, Mingote S, Yetnikoff L, Ztaou S, Velicu V, Rayport S, Chuhma N. Eskenazi D, et al. Front Neural Circuits. 2021 May 19;15:665386. doi: 10.3389/fncir.2021.665386. eCollection 2021. Front Neural Circuits. 2021. PMID: 34093138 Free PMC article. Review. - Postsynaptic integration of glutamatergic and dopaminergic signals in the striatum.
Kötter R. Kötter R. Prog Neurobiol. 1994 Oct;44(2):163-96. doi: 10.1016/0301-0082(94)90037-x. Prog Neurobiol. 1994. PMID: 7831476 Review.
Cited by
- Neuromodulator and neuropeptide sensors and probes for precise circuit interrogation in vivo.
Muir J, Anguiano M, Kim CK. Muir J, et al. Science. 2024 Sep 27;385(6716):eadn6671. doi: 10.1126/science.adn6671. Epub 2024 Sep 27. Science. 2024. PMID: 39325905 Free PMC article. Review. - Codon Usage Bias: A Potential Factor Affecting VGLUT Developmental Expression and Protein Evolution.
Zhao Y, Zhang Y, Feng J, He Z, Li T. Zhao Y, et al. Mol Neurobiol. 2024 Sep 21. doi: 10.1007/s12035-024-04426-8. Online ahead of print. Mol Neurobiol. 2024. PMID: 39305444 - Olfactory Projections to Locomotor Control Centers in the Sea Lamprey.
Beauséjour PA, Veilleux JC, Condamine S, Zielinski BS, Dubuc R. Beauséjour PA, et al. Int J Mol Sci. 2024 Aug 29;25(17):9370. doi: 10.3390/ijms25179370. Int J Mol Sci. 2024. PMID: 39273317 Free PMC article. - Regulation of the Mouse Ventral Tegmental Area by Melanin-Concentrating Hormone.
Spencer CD, Miller PA, Williams-Ikhenoba JG, Nikolova RG, Chee MJ. Spencer CD, et al. J Neurosci. 2024 Jul 3;44(27):e0790232024. doi: 10.1523/JNEUROSCI.0790-23.2024. J Neurosci. 2024. PMID: 38806249 - Prevalent co-release of glutamate and GABA throughout the mouse brain.
Ceballos CC, Ma L, Qin M, Zhong H. Ceballos CC, et al. bioRxiv [Preprint]. 2024 Mar 29:2024.03.27.587069. doi: 10.1101/2024.03.27.587069. bioRxiv. 2024. PMID: 38585864 Free PMC article. Preprint.
References
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. - PubMed
- Bowers CW. Superfluous neurotransmitters? Trends Neurosci. 1994;17:315–320. - PubMed
- Campbell KJ, Takada M, Hattori T. Co-localization of tyrosine hydroxylase and glutamate decarboxylase in a subpopulation of single nigrotectal projection neurons. Brain Res. 1991;558:239–244. - PubMed
- Cheramy A, Leviel V, Glowinski J. Dendritic release of dopamine in the substantia nigra. Nature. 1981;289:537–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous