Type III protein secretion systems in bacterial pathogens of animals and plants - PubMed (original) (raw)
Review
Type III protein secretion systems in bacterial pathogens of animals and plants
C J Hueck. Microbiol Mol Biol Rev. 1998 Jun.
Abstract
Various gram-negative animal and plant pathogens use a novel, sec-independent protein secretion system as a basic virulence mechanism. It is becoming increasingly clear that these so-called type III secretion systems inject (translocate) proteins into the cytosol of eukaryotic cells, where the translocated proteins facilitate bacterial pathogenesis by specifically interfering with host cell signal transduction and other cellular processes. Accordingly, some type III secretion systems are activated by bacterial contact with host cell surfaces. Individual type III secretion systems direct the secretion and translocation of a variety of unrelated proteins, which account for species-specific pathogenesis phenotypes. In contrast to the secreted virulence factors, most of the 15 to 20 membrane-associated proteins which constitute the type III secretion apparatus are conserved among different pathogens. Most of the inner membrane components of the type III secretion apparatus show additional homologies to flagellar biosynthetic proteins, while a conserved outer membrane factor is similar to secretins from type II and other secretion pathways. Structurally conserved chaperones which specifically bind to individual secreted proteins play an important role in type III protein secretion, apparently by preventing premature interactions of the secreted factors with other proteins. The genes encoding type III secretion systems are clustered, and various pieces of evidence suggest that these systems have been acquired by horizontal genetic transfer during evolution. Expression of type III secretion systems is coordinately regulated in response to host environmental stimuli by networks of transcription factors. This review comprises a comparison of the structure, function, regulation, and impact on host cells of the type III secretion systems in the animal pathogens Yersinia spp., Pseudomonas aeruginosa, Shigella flexneri, Salmonella typhimurium, enteropathogenic Escherichia coli, and Chlamydia spp. and the plant pathogens Pseudomonas syringae, Erwinia spp., Ralstonia solanacearum, Xanthomonas campestris, and Rhizobium spp.
Figures
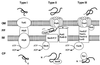
FIG. 1
Schematic overview of the type I, II, and III secretion systems as exemplified by alpha-hemolysin secretion by E. coli (type I), pullulanase secretion by Klebsiella oxytoca (type II), and Yop secretion by Yersinia (type III). OM, outer membrane; PP, periplasm; IM, inner membrane; CP, cytoplasm. ATP hydrolysis by HlyB, SecA, and YscN is indicated. The localization of the secretion signals is shown in the secreted proteins (shaded). N, amino terminus; C, carboxy terminus. For type III secretion, the secretion signal may reside in the 5′-region of the mRNA encoding the secreted protein. Type II and type III secretion involve cytoplasmic chaperones (SecB and Syc, respectively) which bind to presecretory proteins. In type II secretion, the amino-terminal signal sequence is cleaved off by a periplasmic peptidase (LspA) after export of the protein via the sec pathway. Type II and type III secretion share a homologous multimeric outer membrane component (PulD, YscC), while the accessory proteins PulS and VirG, which facilitate outer membrane insertion of PulD and YscC, respectively, differ in the two systems. See the text for further details.
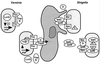
FIG. 2
Selected phenotypic effects of type III secretion pathogenicity mechanisms on host cells and host tissue. (Top left) Panel B shows how Y. pseudotuberculosis injects (translocates) YopH (immunostained, light) into the cytosol of HeLa cells. Translocation of YopH into macrophages results in inhibition of phagocytosis. Panel A shows a type III secretion mutant: YopH is detected only in association with the bacteria. Reprinted with permission from reference . (Top right) Panel B shows the cytotoxic effect of Y. pseudotuberculosis on cultured HeLa cells. Translocation of YopE leads to a collapse of the cytoskeleton. Panel A shows uninfected HeLa cells. Reprinted with permission from reference . (Middle left) Invasion of a polarized HEp-2 epithelial cell via the induction of membrane ruffling by S. typhimurium. This figure has previously appeared on the cover of Mol. Microbiol. 1995, vol. 18 no. 3. Reprinted with permission. (Middle right) Pseudopod (pedestal) formation induced by EPEC on HeLa epithelial cells. Reprinted with permission from reference . (Bottom left) Induction of apoptosis in macrophages infected with S. flexneri. Panel A shows an uninfected macrophage. Panel B shows an apoptotic macrophage infected with wild-type S. flexneri. Panel C shows a macrophage infected with a S. flexneri type III secretion mutant. Reprinted with permission from reference . (Bottom right) Induction of localized tissue necrosis (HR) in a tobacco leaf at sites of infiltration with Erwinia spp. (area 1), buffer alone (area 4), type III secretion mutants (areas 2 and 5), and complemented mutants (areas 3 and 6). Reprinted with permission from reference .
FIG. 3
Homologies between the protein tyrosine phosphatase SptP of S. typhimurium, the cytotoxin YopE and the tyrosine phosphatase YopH from Yersinia spp., and the P. aeruginosa ExoS ADP-ribosyltransferase. Identically shaded boxes indicate regions of sequence similarities. The catalytic cysteine residue in the carboxy-terminal parts of SptP and YopH are shown. The predicted catalytic domain of ExoS is located in the carboxy-terminal quarter of the protein (stippled) (253).
FIG. 4
Similarity of hydrophobic regions (shaded boxes) in the putative membrane translocator proteins YopB and IpaB (173).
FIG. 5
Translocation domains (shaded boxes) of YopE and YopH as determined by reporter gene fusions. The approximate boundaries of the domains are shown in amino acids. Secretion requires the 5′ region of yop genes (codons 1 to 15). The secretion signal may reside in the mRNA (see the text for details).
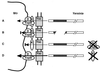
FIG. 6
(A) Secretion and translocation of a Yop from Yersinia spp. into the cytosol of a macrophage (MΦ). (B) Deletion of the translocation domain (ca. aa 15 to 50) results in loss of translocation. (C) YopB and YopD are required for translocation but not for secretion. (D) A YopK mutant exhibits enhanced translocation. See the text for details.
FIG. 7
Schematic representation of the function of the Yop-specific chaperones in secretion. (A) The Yop-specific chaperone binds to the translocation domain of its cognate Yop protein. (B) In the absence of the respective chaperone, secretion of the Yop is impaired. (C) Deletion of the translocation domain abolishes the requirement for the chaperone for secretion. (D) In the absence of the chaperone and YopB/D, secretion is (partially) restored, indicating that the chaperone functions to protect premature interaction of the secreted Yop with YopB/D. See the text for further details.
FIG. 8
Schematic representation of regulation of type III secretion by contact with a eukaryotic cell (shown in the middle) in Yersinia and Shigella spp. For Yersinia spp., prior to cell contact (upper left), the type III secretion channels (Ysc) are kept shut by YopN, and cytoplasmic accumulation of the negative regulatory factor LcrQ leads to transcriptional repression of yop genes (see the text). After cell contact, YopN is released, allowing rapid secretion of LcrQ, which in turn relieves the block from yop gene expression. Anti-host Yop proteins, protected by cognate chaperones (Syc), are translocated into the eukaryotic cell via the type III secretion machinery and YopB/D. For Shigella spp. (only the model of regulation via IpaB/D is shown [see the text for further details]), before contact with a eukaryotic cell (lower right), the S. flexneri type III secretion channels (Mxi and Spa) are kept shut by a complex of IpaB and IpaD. Ipa proteins accumulate in the cytoplasm and are protected from interaction and proteolytic degradation by the chaperone IpgC (C in a triangle). IpaB and IpaC, however, are partially secreted and form a protein-protein complex in the bacterial supernatant. After target cell contact, the IpaB/D block is released and high local concentrations of the IpaB/C complex induce membrane ruffling and uptake of Shigella spp.
FIG. 9
Genetic organization of type III secretion systems and of flagellum biosynthesis genes from B. subtilis and E. coli. Homologies of encoded proteins are indicated by color code (see the text and Fig. 10). The type III secretion systems of animal and plant pathogens are grouped according to genetic similarities. Solid arrows indicate broadly conserved genes, while genes which are conserved only between subgroups are outlined by thicker, colored lines. A thin black outline indicates that no homolog of the respective gene has been identified so far. The filling patterns indicate genes which encode transcription factors (see the text and Table 3). A small s inside a gene symbol indicates secretion of the encoded protein, while the genes which encode chaperonic proteins are labeled ch. Transcriptional units are indicated by arrows underneath the genes where known (see the text for references). For the plant type III secretion systems, the hrc and hrp gene designations are sometimes replaced with c and p, respectively. The genetic maps are drawn according to the references given together with the respective proteins in Fig. 10. See the text for further details.
FIG. 10
Broadly conserved proteins of the type III secretion apparatus and of the flagellum biosynthesis systems in B. subtilis and E. coli/S. typhimurium. The lengths of the proteins in amino acids given underneath the protein designation. Numbers in parentheses indicate references. Subgroups of proteins which exhibit significant similarity are indicated by different grades of shading. See the text for further details. 1, Abbreviations in parentheses denote the organism. Ye, Y. enterocolitica; Ypt, Y. pseudotuberculosis; Yp, Y. pestis. 2, Sequence from Shigella sonnei. In S. flexneri, only part of the respective MxiC sequence is available. 3, Sequence incomplete. 4, In SpaO and other members of the YscQ family, only the carboxy-terminal 80 aa is conserved, while SpaO also shares an internal domain (aa 147 to 194) with SpaO from S. typhimurium. 5, Sequence not available. 6, Older designations are given in parentheses. 7, HrcQB corresponds to the conserved carboxy-terminal domain of other YscQ-family members, while the HrcQA corresponds to the amino-terminal domain of the conserved HrcQ from E. amylovora (Fig. 11). 8, U. Bonas, unpublished. 9, Xcg, X. campestris pv. glycinea. 10, Stm, S. typhimurium; Eco, E. coli. 11, FliM of E. coli is homologous to the amino terminus of B. subtilis FliY. See the text and Fig. 11 for further details. 12, In most cases, the subcellular location is predicted according to the physicochemical properties of the protein or inferred from the location of homologous flagellar biosynthesis proteins. 13, Unified nomenclature for conserved proteins of the type III secretion apparatus. Sct stands for secretion and cellular translocation.
FIG. 11
Schematic representation of homologous domains (shown by corresponding patterns; open areas have no similarity) in YscQ family and flagellar proteins. The N termini of the proteins are at the left side.
FIG. 12
Schematic overview of selected transcriptional regulatory networks in several type III secretion systems. + and − indicate transcriptional activation and repression, respectively. In some cases, regulatory conditions and signals are indicated in parentheses. 2C RR, the protein is a homolog to two-component system response regulators. The modes of action of LcrQ in Yersinia spp. and of OmpR in S. flexneri are unknown. See the text for details.
FIG. 13
Homologies between type III secretory proteins and factors which form the flagellum-specific export pathway. Part of the Yersinia type III secretion gene cluster is shown in comparison to the flagellar E. coli genes and the location of the respective E. coli proteins in the flagellum basal body. Homologies are indicated by the color code. OM, outer membrane; PP, periplasmic space; CM, cytoplasmic membrane. ATP hydrolysis by the cytoplasmic ATPase FliI is indicated.
FIG. 14
Conserved domains in selected proteins of the large GspD family. The generally conserved domains are shown in black and are aligned with each other, while domains which are conserved in subfamilies are shown in corresponding shadings. Kpn, Klebsiella pneumoniae; Ech, Erwinia chrysanthemi; BP, bacteriophage; Hin, Haemophilus influenzae; Yen, Yersinia enterocolitica; Sfl, Shigella flexneri.
Similar articles
- Detection of type III secretion genes as a general indicator of bacterial virulence.
Stuber K, Frey J, Burnens AP, Kuhnert P. Stuber K, et al. Mol Cell Probes. 2003 Feb;17(1):25-32. doi: 10.1016/s0890-8508(02)00108-1. Mol Cell Probes. 2003. PMID: 12628591 - The Xanthomonas Hrp type III system secretes proteins from plant and mammalian bacterial pathogens.
Rossier O, Wengelnik K, Hahn K, Bonas U. Rossier O, et al. Proc Natl Acad Sci U S A. 1999 Aug 3;96(16):9368-73. doi: 10.1073/pnas.96.16.9368. Proc Natl Acad Sci U S A. 1999. PMID: 10430949 Free PMC article. - Type III secretion machines: bacterial devices for protein delivery into host cells.
Galán JE, Collmer A. Galán JE, et al. Science. 1999 May 21;284(5418):1322-8. doi: 10.1126/science.284.5418.1322. Science. 1999. PMID: 10334981 Review. - The bacterial injection kit: type III secretion systems.
Mota LJ, Cornelis GR. Mota LJ, et al. Ann Med. 2005;37(4):234-49. doi: 10.1080/07853890510037329. Ann Med. 2005. PMID: 16019722 Review. - Subterfuge and manipulation: type III effector proteins of phytopathogenic bacteria.
Grant SR, Fisher EJ, Chang JH, Mole BM, Dangl JL. Grant SR, et al. Annu Rev Microbiol. 2006;60:425-49. doi: 10.1146/annurev.micro.60.080805.142251. Annu Rev Microbiol. 2006. PMID: 16753033 Review.
Cited by
- Proteus faecis: a potentially pathogenic bacterium isolated from the freshwater Yangtze finless porpoise.
McLaughlin RW, Wang Y, Zhang S, Xie H, Wan X, Liu H, Hao Y, Wang C, Zheng J. McLaughlin RW, et al. Antonie Van Leeuwenhoek. 2024 Sep 21;118(1):7. doi: 10.1007/s10482-024-02023-2. Antonie Van Leeuwenhoek. 2024. PMID: 39305395 - Novel humanized anti-PcrV monoclonal antibody COT-143 protects mice from lethal Pseudomonas aeruginosa infection via inhibition of toxin translocation by the type III secretion system.
Numata S, Hara T, Izawa M, Okuno Y, Sato Y, Yamane S, Maki H, Sato T, Yamano Y. Numata S, et al. Antimicrob Agents Chemother. 2024 Oct 8;68(10):e0069424. doi: 10.1128/aac.00694-24. Epub 2024 Sep 13. Antimicrob Agents Chemother. 2024. PMID: 39269189 Free PMC article. - Salmonella Typhimurium exploits host polyamines for assembly of the type 3 secretion machinery.
Miki T, Uemura T, Kinoshita M, Ami Y, Ito M, Okada N, Furuchi T, Kurihara S, Haneda T, Minamino T, Kim YG. Miki T, et al. PLoS Biol. 2024 Aug 5;22(8):e3002731. doi: 10.1371/journal.pbio.3002731. eCollection 2024 Aug. PLoS Biol. 2024. PMID: 39102375 Free PMC article. - Evaluating the Safety of Bacillus cereus GW-01 Obtained from Sheep Rumen Chyme.
Xu B, Huang X, Qin H, Lei Y, Zhao S, Liu S, Liu G, Zhao J. Xu B, et al. Microorganisms. 2024 Jul 18;12(7):1457. doi: 10.3390/microorganisms12071457. Microorganisms. 2024. PMID: 39065225 Free PMC article. - An improved bacterial mRNA enrichment strategy in dual RNA sequencing to unveil the dynamics of plant-bacterial interactions.
Shilpha J, Lee J, Kwon JS, Lee HA, Nam JY, Jang H, Kang WH. Shilpha J, et al. Plant Methods. 2024 Jul 1;20(1):99. doi: 10.1186/s13007-024-01227-x. Plant Methods. 2024. PMID: 38951818 Free PMC article.
References
- Abeles A L, Friedman S A, Austin S J. Partition of unit-copy miniplasmids to daughter cells. III. The DNA sequence and functional organization of the P1 partition region. J Mol Biol. 1985;185:261–272. - PubMed
- Adler B, Sasakawa C, Tobe T, Makino S, Komatsu K, Yoshikawa M. A dual transcriptional activation system for the 230 kb plasmid genes coding for virulence-associated antigens of Shigella flexneri. Mol Microbiol. 1989;3:627–635. - PubMed
- Aizawa S-I. Flagellar assembly in Salmonella typhimurium. Mol Microbiol. 1996;19:1–5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous