Interacting helical faces of subunits a and c in the F1Fo ATP synthase of Escherichia coli defined by disulfide cross-linking - PubMed (original) (raw)
Interacting helical faces of subunits a and c in the F1Fo ATP synthase of Escherichia coli defined by disulfide cross-linking
W Jiang et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Subunits a and c of Fo are thought to cooperatively catalyze proton translocation during ATP synthesis by the Escherichia coli F1Fo ATP synthase. Optimizing mutations in subunit a at residues A217, I221, and L224 improves the partial function of the cA24D/cD61G double mutant and, on this basis, these three residues were proposed to lie on one face of a transmembrane helix of subunit a, which then interacted with the transmembrane helix of subunit c anchoring the essential aspartyl group. To test this model, in the present work Cys residues were introduced into the second transmembrane helix of subunit c and the predicted fourth transmembrane helix of subunit a. After treating the membrane vesicles of these mutants with Cu(1, 10-phenanthroline)2SO4 at 0 degrees, 10 degrees, or 20 degreesC, strong a-c dimer formation was observed at all three temperatures in membranes of 7 of the 65 double mutants constructed, i.e., in the aS207C/cI55C, aN214C/cA62C, aN214C/cM65C, aI221C/cG69C, aI223C/cL72C, aL224C/cY73C, and aI225C/cY73C double mutant proteins. The pattern of cross-linking aligns the helices in a parallel fashion over a span of 19 residues with the aN214C residue lying close to the cA62C and cM65C residues in the middle of the membrane. Lesser a-c dimer formation was observed in nine other double mutants after treatment at 20 degreesC in a pattern generally supporting that indicated by the seven landmark residues cited above. Cross-link formation was not observed between helix-1 of subunit c and helix-4 of subunit a in 19 additional combinations of doubly Cys-substituted proteins. These results provide direct chemical evidence that helix-2 of subunit c and helix-4 of subunit a pack close enough to each other in the membrane to interact during function. The proximity of helices supports the possibility of an interaction between Arg210 in helix-4 of subunit a and Asp61 in helix-2 of subunit c during proton translocation, as has been suggested previously.
Figures
Figure 1
Growth (function) of Cys-substituted mutants (A) and summary of a–c cross-linking results (B). (A) Cells were plated on succinate minimal medium and the colony size (mm) was measured after incubation at 37°C for 3 days as a measure of oxidative phosphorylation function. The chromosomal, wild-type control strain showed colonies of 2 mm after 3 days at 37°C. (B) Relative yield of a–c cross-linked dimer formed between Cys at the positions indicated. Membrane vesicles were treated with CuP at 10°C for 60 min. Relative yield of the a–c cross-link product: 0, none; −/+, ≤5%; +, 6–10%; ++, 11–20%; +++, 20–40%; ++++, >40%. 0* indicates Cys pair forming no cross-link at 10°C but significant cross-link (8%) at 20°C.
Figure 2
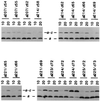
Cross-linking of _a_I221C-substituted membranes in varying combinations with second Cys in subunit c. SDS-solubilized membranes were electrophoresed under nonreducing condition before immunoblotting. The blot was first probed with antiserum against subunit c. The blot was then stripped of bound antibodies by submerging it in a buffer containing 62.5 mM Tris⋅HCl (pH 6.8), 2% SDS, and 100 mM 2-mercaptoethanol for 30 min at 50°C and reprobed with antiserum against subunit a. The bands marked IA* are immunoblotting artifacts. Wild-type membranes are from pDF163- (uncBEFH+) transformed strain JWP109 (ΔuncB–H). Deletion membranes are from strain JWP109 (ΔuncB–H).
Figure 3
Comparison of a–c cross-link formation at 10 and 20°C with a series of doubly Cys-substituted membranes. A portion of an immunoblot of a SDS gel run under nonreducing conditions is shown following probing with antiserum to subunit a. The positions of the double Cys substitutions and temperature (10°C or 20°C) of the 1-h CuP cross-linking reaction are indicated.
Figure 4
Formation of a–c cross-links by CuP reaction at 10°C in a series of doubly Cys-substituted membranes. The conditions are as described in Fig. 3. The positions of the double Cys substitutions are indicated. Wild-type membranes are from a pDF163 transformant of strain JWP109 (ΔuncB–H). Deletion membranes are from strain JWP109 (ΔuncB–H).
Figure 5
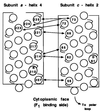
Summary of major cross-links formed between helix-4 of subunit a and helix-2 of subunit c at 10°C. The circles numbered without specifying residue type indicate the position of the Cys substitutions in α-helical representations of the transmembrane segments. The positions of essential residues _a_R210 and _c_D61 are also indicated.
Figure 6
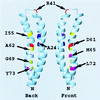
Ribbon depiction of the folding of subunit c derived from the NMR structure. The “front” and “back” face of two subunits c are indicated. A functional _c_2 dimer is proposed to be formed by packing the front face of one monomer against the back face of a second monomer (46), i.e., with D61 of one monomer packed between A24 and A62 of a second monomer. The positions of the backbone atoms of key residues discussed in the text are indicated. The illustration was done in the program
molmol
(45).
Similar articles
- Arrangement of the multicopy H+-translocating subunit c in the membrane sector of the Escherichia coli F1F0 ATP synthase.
Jones PC, Jiang W, Fillingame RH. Jones PC, et al. J Biol Chem. 1998 Jul 3;273(27):17178-85. doi: 10.1074/jbc.273.27.17178. J Biol Chem. 1998. PMID: 9642286 - Structure of the membrane domain of subunit b of the Escherichia coli F0F1 ATP synthase.
Dmitriev O, Jones PC, Jiang W, Fillingame RH. Dmitriev O, et al. J Biol Chem. 1999 May 28;274(22):15598-604. doi: 10.1074/jbc.274.22.15598. J Biol Chem. 1999. PMID: 10336456 - Structural model of the transmembrane Fo rotary sector of H+-transporting ATP synthase derived by solution NMR and intersubunit cross-linking in situ.
Fillingame RH, Dmitriev OY. Fillingame RH, et al. Biochim Biophys Acta. 2002 Oct 11;1565(2):232-45. doi: 10.1016/s0005-2736(02)00572-2. Biochim Biophys Acta. 2002. PMID: 12409198 Review. - H+ transport and coupling by the F0 sector of the ATP synthase: insights into the molecular mechanism of function.
Fillingame RH. Fillingame RH. J Bioenerg Biomembr. 1992 Oct;24(5):485-91. doi: 10.1007/BF00762366. J Bioenerg Biomembr. 1992. PMID: 1331039 Review.
Cited by
- Arg-735 of the 100-kDa subunit a of the yeast V-ATPase is essential for proton translocation.
Kawasaki-Nishi S, Nishi T, Forgac M. Kawasaki-Nishi S, et al. Proc Natl Acad Sci U S A. 2001 Oct 23;98(22):12397-402. doi: 10.1073/pnas.221291798. Epub 2001 Oct 9. Proc Natl Acad Sci U S A. 2001. PMID: 11592980 Free PMC article. - Polar residues drive association of polyleucine transmembrane helices.
Zhou FX, Merianos HJ, Brunger AT, Engelman DM. Zhou FX, et al. Proc Natl Acad Sci U S A. 2001 Feb 27;98(5):2250-5. doi: 10.1073/pnas.041593698. Epub 2001 Feb 13. Proc Natl Acad Sci U S A. 2001. PMID: 11226225 Free PMC article. - Clostridium pasteurianum F1Fo ATP synthase: operon, composition, and some properties.
Das A, Ljungdahl LG. Das A, et al. J Bacteriol. 2003 Sep;185(18):5527-35. doi: 10.1128/JB.185.18.5527-5535.2003. J Bacteriol. 2003. PMID: 12949105 Free PMC article. - Direct observation of stepped proteolipid ring rotation in E. coli F₀F₁-ATP synthase.
Ishmukhametov R, Hornung T, Spetzler D, Frasch WD. Ishmukhametov R, et al. EMBO J. 2010 Dec 1;29(23):3911-23. doi: 10.1038/emboj.2010.259. Epub 2010 Oct 29. EMBO J. 2010. PMID: 21037553 Free PMC article. - Structure analysis of membrane-reconstituted subunit c-ring of E. coli H+-ATP synthase by solid-state NMR.
Todokoro Y, Kobayashi M, Sato T, Kawakami T, Yumen I, Aimoto S, Fujiwara T, Akutsu H. Todokoro Y, et al. J Biomol NMR. 2010 Sep;48(1):1-11. doi: 10.1007/s10858-010-9432-x. Epub 2010 Jul 2. J Biomol NMR. 2010. PMID: 20596883
References
- Boyer P D. Annu Rev Biochem. 1997;66:717–749. - PubMed
- Deckers-Hebestreit G, Altendorf K. Annu Rev Microbiol. 1996;50:791–824. - PubMed
- Fillingame R H. J Exp Biol. 1997;200:217–224. - PubMed
- Fillingame R H. The Bacteria. Vol. 12. New York: Academic; 1990. pp. 345–391.
- Abrahams J P, Leslie A G, Lutter R, Walker J E. Nature (London) 1994;370:621–628. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources