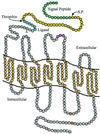Cloning and characterization of human protease-activated receptor 4 - PubMed (original) (raw)
Cloning and characterization of human protease-activated receptor 4
W F Xu et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Protease-activated receptors 1-3 (PAR1, PAR2, and PAR3) are members of a unique G protein-coupled receptor family. They are characterized by a tethered peptide ligand at the extracellular amino terminus that is generated by minor proteolysis. A partial cDNA sequence of a fourth member of this family (PAR4) was identified in an expressed sequence tag database, and the full-length cDNA clone has been isolated from a lymphoma Daudi cell cDNA library. The ORF codes for a seven transmembrane domain protein of 385 amino acids with 33% amino acid sequence identity with PAR1, PAR2, and PAR3. A putative protease cleavage site (Arg-47/Gly-48) was identified within the extracellular amino terminus. COS cells transiently transfected with PAR4 resulted in the formation of intracellular inositol triphosphate when treated with either thrombin or trypsin. A PAR4 mutant in which the Arg-47 was replaced with Ala did not respond to thrombin or trypsin. A hexapeptide (GYPGQV) representing the newly exposed tethered ligand from the amino terminus of PAR4 after proteolysis by thrombin activated COS cells transfected with either wild-type or the mutant PAR4. Northern blot showed that PAR4 mRNA was expressed in a number of human tissues, with high levels being present in lung, pancreas, thyroid, testis, and small intestine. By fluorescence in situ hybridization, the human PAR4 gene was mapped to chromosome 19p12.
Figures
Figure 1
Nucleotide and deduced amino acid sequences for human PAR4. The nucleotide sequence of the 4.9-kb PAR4 cDNA was determined. The amino acid sequence encoded by the longest ORF is shown. Consensus polyadenylation signals are underlined.
Figure 2
Diagram illustrating the proposed seven transmembrane-domain organization for PAR4. The signal peptide is shown in green; the amino-terminal peptide cleaved by thrombin is in yellow; the tethered peptide ligand is in blue; the seven transmembrane-domain regions are in gold; the remaining extracellular and intracellular regions are shown in gray. The CHD sequence in the second transmembrane loop that is present in the four known PAR proteins is shown in pink. A potential serine phosphorylation site for CK II in the sequence SGR and a potential phosphorylation site for protein kinase II in the sequence SPGD are shown in orange (21). An attached Y refers to a potential N-linked glycosylation site, and S.P. refers to signal peptidase.
Figure 3
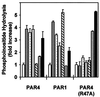
Agonist activity of thrombin, trypsin, and activating peptides on COS cells expressing PAR1, PAR4, or PAR4 protease cleavage site mutant (R47A). Bars: open, control; upward hatched, thrombin (100 nM); stippled, γ-thrombin (100 nM); shaded, trypsin (100 nM); downward hatched, PAR1 activation peptide (100 μM); horizontally hatched, PAR4 activation peptide (100 μM); solid, PAR4 activation peptide (500 μM).
Figure 4
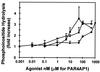
Dose-dependent response of various agonists on PAR4. Concentration of proteases is presented as nanomolar, whereas peptide concentrations are presented as micromolar. EC50 values are estimated at 5 nM for thrombin and trypsin and about 100 μM for PAR4-activating peptide. ○, Thrombin; ▪, trypsin; ♦, PAR4 activation peptide.
Figure 5
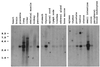
Northern blot of human multiple tissue mRNA hybridized to a human PAR4 cDNA probe. Tissue sources are given at the top and the positions of sized markers (in kilobases) are indicated on the left. The predominate hybridizing species corresponds to an mRNA of about 2.7 kb.
Similar articles
- Neutrophil proteases can inactivate human PAR3 and abolish the co-receptor function of PAR3 on murine platelets.
Cumashi A, Ansuini H, Celli N, De Blasi A, O'Brien PJ, Brass LF, Molino M. Cumashi A, et al. Thromb Haemost. 2001 Mar;85(3):533-8. Thromb Haemost. 2001. PMID: 11307827 - Proteolysis of the exodomain of recombinant protease-activated receptors: prediction of receptor activation or inactivation by MALDI mass spectrometry.
Loew D, Perrault C, Morales M, Moog S, Ravanat C, Schuhler S, Arcone R, Pietropaolo C, Cazenave JP, van Dorsselaer A, Lanza F. Loew D, et al. Biochemistry. 2000 Sep 5;39(35):10812-22. doi: 10.1021/bi0003341. Biochemistry. 2000. PMID: 10978167 - Protease-activated receptor 1 (PAR1) and PAR4 heterodimers are required for PAR1-enhanced cleavage of PAR4 by α-thrombin.
Arachiche A, Mumaw MM, de la Fuente M, Nieman MT. Arachiche A, et al. J Biol Chem. 2013 Nov 8;288(45):32553-32562. doi: 10.1074/jbc.M113.472373. Epub 2013 Oct 4. J Biol Chem. 2013. PMID: 24097976 Free PMC article. - Protease-activated receptor 4: a critical participator in inflammatory response.
Fu Q, Cheng J, Gao Y, Zhang Y, Chen X, Xie J. Fu Q, et al. Inflammation. 2015 Apr;38(2):886-95. doi: 10.1007/s10753-014-9999-6. Inflammation. 2015. PMID: 25120239 Review. - Cofactoring and dimerization of proteinase-activated receptors.
Lin H, Liu AP, Smith TH, Trejo J. Lin H, et al. Pharmacol Rev. 2013 Sep 24;65(4):1198-213. doi: 10.1124/pr.111.004747. Print 2013. Pharmacol Rev. 2013. PMID: 24064459 Free PMC article. Review.
Cited by
- Protease-activated receptor (PAR) 1 and PAR4 differentially regulate factor V expression from human platelets.
Duvernay M, Young S, Gailani D, Schoenecker J, Hamm HE. Duvernay M, et al. Mol Pharmacol. 2013 Apr;83(4):781-92. doi: 10.1124/mol.112.083477. Epub 2013 Jan 10. Mol Pharmacol. 2013. PMID: 23307185 Free PMC article. - Protease-Activated Receptors in the Intestine: Focus on Inflammation and Cancer.
Sébert M, Sola-Tapias N, Mas E, Barreau F, Ferrand A. Sébert M, et al. Front Endocrinol (Lausanne). 2019 Oct 24;10:717. doi: 10.3389/fendo.2019.00717. eCollection 2019. Front Endocrinol (Lausanne). 2019. PMID: 31708870 Free PMC article. Review. - Initiation of human colon cancer cell proliferation by trypsin acting at protease-activated receptor-2.
Darmoul D, Marie JC, Devaud H, Gratio V, Laburthe M. Darmoul D, et al. Br J Cancer. 2001 Sep 1;85(5):772-9. doi: 10.1054/bjoc.2001.1976. Br J Cancer. 2001. PMID: 11531266 Free PMC article. - Design, synthesis, and biological characterization of a peptide-mimetic antagonist for a tethered-ligand receptor.
Andrade-Gordon P, Maryanoff BE, Derian CK, Zhang HC, Addo MF, Darrow AL, Eckardt AJ, Hoekstra WJ, McComsey DF, Oksenberg D, Reynolds EE, Santulli RJ, Scarborough RM, Smith CE, White KB. Andrade-Gordon P, et al. Proc Natl Acad Sci U S A. 1999 Oct 26;96(22):12257-62. doi: 10.1073/pnas.96.22.12257. Proc Natl Acad Sci U S A. 1999. PMID: 10535908 Free PMC article. - Glycosylation and the activation of proteinase-activated receptor 2 (PAR(2)) by human mast cell tryptase.
Compton SJ, Renaux B, Wijesuriya SJ, Hollenberg MD. Compton SJ, et al. Br J Pharmacol. 2001 Oct;134(4):705-18. doi: 10.1038/sj.bjp.0704303. Br J Pharmacol. 2001. PMID: 11606310 Free PMC article.
References
- Scher W. Lab Invest. 1987;57:607–633. - PubMed
- Vu T K H, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. - PubMed
- Rasmussen U B, Vouret-Craviari V, Jallat S, Schlesinger Y, Pages G, Pavirani A, Lecocq J P, Pouyssegur J, Van Obberghen-Schilling E. FEBS Lett. 1991;288:123–128. - PubMed
- Ishihara H, Connolly A J, Zeng D, Kahn M L, Zheng Y W, Timmons C, Tram T, Coughlin S R. Nature (London) 1997;356:502–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials