Quorum sensing in Escherichia coli and Salmonella typhimurium - PubMed (original) (raw)
Quorum sensing in Escherichia coli and Salmonella typhimurium
M G Surette et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Escherichia coli and Salmonella typhimurium strains grown in Luria-Bertani medium containing glucose secrete a small soluble heat labile organic molecule that is involved in intercellular communication. The factor is not produced when the strains are grown in Luria-Bertani medium in the absence of glucose. Maximal secretion of the substance occurs in midexponential phase, and the extracellular activity is degraded as the glucose is depleted from the medium or by the onset of stationary phase. Destruction of the signaling molecule in stationary phase indicates that, in contrast to other quorum-sensing systems, quorum sensing in E. coli and S. typhimurium is critical for regulating behavior in the prestationary phase of growth. Our results further suggest that the signaling factor produced by E. coli and S. typhimurium is used to communicate both the cell density and the metabolic potential of the environment. Several laboratory and clinical strains of E. coli and S. typhimurium were screened for production of the signaling molecule, and most strains make it under conditions similar to those shown here for E. coli AB1157 and S. typhimurium LT2. However, we also show that E. coli strain DH5alpha does not make the soluble factor, indicating that this highly domesticated strain has lost the gene(s) or biosynthetic machinery necessary to produce the signaling substance. Implications for the involvement of quorum sensing in pathogenesis are discussed.
Figures
Figure 1
E. coli AB1157 and S. typhimurium LT2 cell-free culture fluids contain a signaling substance that induces luminescence in V. harveyi. The responses of V. harveyi reporter strains BB170 (sensor 1−, sensor 2+) (A), and BB886 (sensor 1+, sensor 2−) (B) to signaling substances present in cell-free culture fluids from E. coli, S. typhimurium, and V. harveyi strains are shown. A bright culture of each reporter strain was diluted 1:5,000 into fresh medium, and the light production per cell then was measured during the growth of the diluted culture. Cell-free culture fluids or sterile growth medium were added at a final concentration of 10% (vol/vol) at the start of the experiment. The data for the 5-hr time point are shown and are presented as the percent of the activity obtained when V. harveyi cell-free spent culture fluids are added. V.h, V. harveyi; S.t, S. typhimurium, and E.c, E. coli.
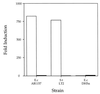
Figure 2
Viable E. coli and S. typhimurium actively secrete the signaling molecule. The response of the V. harveyi reporter strain BB170 (sensor 1−, sensor 2+) to a signaling substance produced and secreted by E. coli AB1157 and S. typhimurium LT2 but not E. coli DH5α is shown. V. harveyi reporter strain BB170 was diluted 1:5,000 in AB medium and light output per cell was monitored during growth. At the start of the experiment, either 1 × 106 E. coli AB1157, S. typhimurium LT2, or E. coli DH5α washed and resuspended viable cells (empty bars) or UV-killed cells (filled bars) was added. The data are presented as the fold-activation above the endogenous level of luminescence expressed by V. harveyi BB170 at the 5-hr time point. S.t, S. typhimurium; E.c, E. coli. Results for replicates were within 10%.
Figure 3
Effect of glucose depletion on the production and degradation of the signaling activity by S. typhimurium LT2. S. typhimurium LT2 was grown in LB medium containing either 0.1% glucose (A) or 0.5% glucose (B). At the specified times cell-free culture fluids were prepared and assayed for signaling activity in the luminescence stimulation assay (bars), and the concentration of glucose remaining (○). The cell number was determined at each time by diluting and plating the S. typhimurium LT2 on LB medium and counting colonies the next day (□). The signaling activity is presented as the percent of the activity obtained when V. harveyi cell-free spent culture fluids are added. These data correspond to the 5-hr time point in the luminescence stimulation assay. The glucose concentration is shown as % glucose remaining. Cell number is cells/ml × 10−9. ⑊ indicates that the time axis is not drawn to scale after 8 hr. Replicate samples agreed within 10%.
Similar articles
- Cell-to-cell communication in Escherichia coli and Salmonella typhimurium: they may be talking, but who's listening?
Fuqua C, Greenberg EP. Fuqua C, et al. Proc Natl Acad Sci U S A. 1998 Jun 9;95(12):6571-2. doi: 10.1073/pnas.95.12.6571. Proc Natl Acad Sci U S A. 1998. PMID: 9618451 Free PMC article. No abstract available. - Regulation of autoinducer production in Salmonella typhimurium.
Surette MG, Bassler BL. Surette MG, et al. Mol Microbiol. 1999 Jan;31(2):585-95. doi: 10.1046/j.1365-2958.1999.01199.x. Mol Microbiol. 1999. PMID: 10027975 - Absence of association of autoinducer-2-based quorum sensing with heat and acid resistance of Salmonella.
Yoon Y, Sofos JN. Yoon Y, et al. J Food Sci. 2010 Sep;75(7):M444-8. doi: 10.1111/j.1750-3841.2010.01744.x. Epub 2010 Sep 2. J Food Sci. 2010. PMID: 21535554 - Quorum sensing in Escherichia coli and Salmonella.
Walters M, Sperandio V. Walters M, et al. Int J Med Microbiol. 2006 Apr;296(2-3):125-31. doi: 10.1016/j.ijmm.2006.01.041. Epub 2006 Feb 17. Int J Med Microbiol. 2006. PMID: 16487745 Review. - Eavesdropping by bacteria: the role of SdiA in Escherichia coli and Salmonella enterica serovar Typhimurium quorum sensing.
Smith JL, Fratamico PM, Yan X. Smith JL, et al. Foodborne Pathog Dis. 2011 Feb;8(2):169-78. doi: 10.1089/fpd.2010.0651. Epub 2010 Oct 30. Foodborne Pathog Dis. 2011. PMID: 21034261 Review.
Cited by
- Cinnamon Oil Inhibits Shiga Toxin Type 2 Phage Induction and Shiga Toxin Type 2 Production in Escherichia coli O157:H7.
Sheng L, Rasco B, Zhu MJ. Sheng L, et al. Appl Environ Microbiol. 2016 Oct 27;82(22):6531-6540. doi: 10.1128/AEM.01702-16. Print 2016 Nov 15. Appl Environ Microbiol. 2016. PMID: 27590808 Free PMC article. - Autoinducer 2 production by Streptococcus gordonii DL1 and the biofilm phenotype of a luxS mutant are influenced by nutritional conditions.
Blehert DS, Palmer RJ Jr, Xavier JB, Almeida JS, Kolenbrander PE. Blehert DS, et al. J Bacteriol. 2003 Aug;185(16):4851-60. doi: 10.1128/JB.185.16.4851-4860.2003. J Bacteriol. 2003. PMID: 12897005 Free PMC article. - luxS and arcB control aerobic growth of Actinobacillus actinomycetemcomitans under iron limitation.
Fong KP, Gao L, Demuth DR. Fong KP, et al. Infect Immun. 2003 Jan;71(1):298-308. doi: 10.1128/IAI.71.1.298-308.2003. Infect Immun. 2003. PMID: 12496179 Free PMC article. - Modified _N_-acyl-L-homoserine lactone compounds abrogate Las-dependent quorum-sensing response in human pathogen Pseudomonas aeruginosa.
Ballante F, Turkina MV, Ntzouni M, Magnusson KE, Vikström E. Ballante F, et al. Front Mol Biosci. 2023 Oct 16;10:1264773. doi: 10.3389/fmolb.2023.1264773. eCollection 2023. Front Mol Biosci. 2023. PMID: 37908228 Free PMC article. - Autoinducer-2 is produced in saliva-fed flow conditions relevant to natural oral biofilms.
Rickard AH, Campagna SR, Kolenbrander PE. Rickard AH, et al. J Appl Microbiol. 2008 Dec;105(6):2096-103. doi: 10.1111/j.1365-2672.2008.03910.x. J Appl Microbiol. 2008. PMID: 19120655 Free PMC article.
References
- Engebrecht J, Nealson K, Silverman M. Cell. 1983;32:773–781. - PubMed
- Bassler B L, Silverman M R. In: Two-Component Signal Transduction. Hoch J A, Silhavy T J, editors. Washington, DC: Am. Soc. Microbiol.; 1995. pp. 431–445.
- Kaplan H B, Plamam L. FEMS Microbiol Lett. 1996;139:89–95. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources