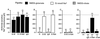Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+ - PubMed (original) (raw)
Nedd4 mediates control of an epithelial Na+ channel in salivary duct cells by cytosolic Na+
A Dinudom et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Epithelial Na+ channels are expressed widely in absorptive epithelia such as the renal collecting duct and the colon and play a critical role in fluid and electrolyte homeostasis. Recent studies have shown that these channels interact via PY motifs in the C terminals of their alpha, beta, and gamma subunits with the WW domains of the ubiquitin-protein ligase Nedd4. Mutation or deletion of these PY motifs (as occurs, for example, in the heritable form of hypertension known as Liddle's syndrome) leads to increased Na+ channel activity. Thus, binding of Nedd4 by the PY motifs would appear to be part of a physiological control system for down-regulation of Na+ channel activity. The nature of this control system is, however, unknown. In the present paper, we show that Nedd4 mediates the ubiquitin-dependent down-regulation of Na+ channel activity in response to increased intracellular Na+. We further show that Nedd4 operates downstream of Go in this feedback pathway. We find, however, that Nedd4 is not involved in the feedback control of Na+ channels by intracellular anions. Finally, we show that Nedd4 has no influence on Na+ channel activity when the Na+ and anion feedback systems are inactive. We conclude that Nedd4 normally mediates feedback control of epithelial Na+ channels by intracellular Na+, and we suggest that the increased Na+ channel activity observed in Liddle's syndrome is attributable to the loss of this regulatory feedback system.
Figures
Figure 1
Expression of Nedd4 protein in the mouse mandibular gland. Sections of the formalin-fixed, paraffin-embedded mandibular gland tissue were subjected to immunohistochemical analysis by using either the pre-immune serum (A and C), or a 1:200 dilution of anti-Nedd4 serum (B and D). Note the strong expression of Nedd4 (brown) in all granular duct cells. [Bars = 160 μm (A and B), or 80 μm (C and D).]
Figure 2
(A) The effects of the inclusion in NMDG-glutamate pipette solution of the GST–WW fusion protein (G-W), GST control (G), anti-Nedd4 IgG (A-Nd4), or pre-immune IgG on the chord conductance measured at −80 mV of the amiloride-sensitive Na+ conductance. (B) The effects of the inclusion in 72 mmol/l Na+ pipette solution of the GST control, the GST–WW fusion protein, pre-immune IgG, or anti-Nedd4 IgG on the chord conductance measured at −80 mV of the amiloride-sensitive Na+ conductance. (C) The effects of the inclusion in NMDG–NO3 pipette solution of the GST–WW fusion protein or anti-Nedd4 antibody on the chord conductance measured at −80 mV of the amiloride-sensitive Na+ conductance. (D) The effects of the inclusion of activated α subunit of Go(αo), activated α subunit of Go plus pre-immune IgG, activated α subunit of Go plus anti-Nedd4 IgG, or activated α subunit of Gi2 plus anti-Nedd4 IgG in the NMDG-glutamate pipette solution on the chord conductance measured at −80 mV of the amiloride-sensitive Na+ conductance. The chord conductance observed by using the NMDG-glutamate pipette solution is shown in each panel as a dotted line.
Figure 3
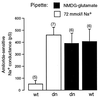
The effects of the inclusion in the 72 mmol/l Na+ pipette solution or in the NMDG-glutamate pipette solution of the GST–dn–ubiquitin (K48R) fusion protein (dn) and the GST–wt–ubiquitin fusion protein (wt) on the chord conductance measured at −80 mV of the amiloride-sensitive Na+ conductance.
Figure 4
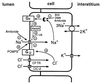
Proposed model for feedback regulation of Na+ channels in salivary duct cells by cytosolic Na+ and Cl− acting through G proteins and Nedd4.
References
- Garty H, Palmer L G. Physiol Rev. 1997;77:359–396. - PubMed
- Dinudom A, Young J A, Cook D I. Pflügers Arch. 1993;423:164–166. - PubMed
- Lifton R P. Science. 1996;272:676–680. - PubMed
- Canessa C M, Schild I, Buell G, Thorens B, Gautschi I, Horisberger J D, Rossier B C. Nature (London) 1994;367:463–467. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources