p107 is a suppressor of retinoblastoma development in pRb-deficient mice - PubMed (original) (raw)
p107 is a suppressor of retinoblastoma development in pRb-deficient mice
E Robanus-Maandag et al. Genes Dev. 1998.
Abstract
Hemizygosity for the retinoblastoma gene RB in man strongly predisposes to retinoblastoma. In the mouse, however, Rb hemizygosity leaves the retina normal, whereas in Rb-/- chimeras pRb-deficient retinoblasts undergo apoptosis. To test whether concomitant inactivation of the Rb-related gene p107 is required to unleash the oncogenic potential of pRb deficiency in the mouse retina, we inactivated both Rb and p107 by homologous recombination in embryonic stem cells and generated chimeric mice. Retinoblastomas were found in five out of seven adult pRb/p107-deficient chimeras. The retinal tumors showed amacrine cell differentiation, and therefore originated from cells committed to the inner but not the outer nuclear layer. Retinal lesions were already observed at embryonic day 17.5. At this stage, the primitive nuclear layer exhibited severe dysplasia, including rosette-like arrangements, and apoptosis. These findings provide formal proof for the role of loss of Rb in retinoblastoma development in the mouse and the first in vivo evidence that p107 can exert a tumor suppressor function.
Figures
Figure 1
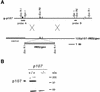
Targeted disruption of p107. (A) Restriction map of the wild-type p107 allele around codon 145 at the Eco_RV site and DNA targeting construct. Black boxes indicate determined exons. Upon homologous recombination a fusion transcript is generated of p107, truncated behind codon 145, with IRES_βgeo. Probe A and probe B detect modifications at p107. (B) Western blot analysis of p107 in lysates prepared from p107 +/+ and p107 −/− ES cells. Positions of molecular mass standards and p107 are indicated.
Figure 2
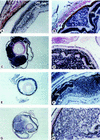
Retinoblastoma in chimeric Rb−/−; p107 −/−(;hIRBP_p53DD_) mice. Histological sections of eyes, stained with hematoxilyn-eosin. (A) Dysplasia in the transit region of the inner nuclear to the outer plexiform retinal layer of a chimeric Rb+/−;p107 −/− adult mouse (5 months). (B) Developing retinal tumor consisting of inner-nuclear-layer-like cells growing between the outer nuclear layer and the RPE in a chimeric Rb−/−;p107 −/− mouse (1 month). (C) Large retinoblastoma in a chimeric Rb−/−;p107 −/− mouse (3.5 months), with (D) rosettes consisting of rearranged photoreceptor cells (open arrows) and tumor cells (solid arrows). (E) Malignant retinoblastoma, with (F) nodular growth of the inner nuclear layer disrupting the outer nuclear layer in a young chimeric Rb−/−;p107 −/−; hIRBP_p53DD_ mouse (P15). (G) Large retinoblasto ma in a chimeric Rb−/−;p107 −/−;hIRBP_p53DD_ mouse (2.5 months), with (H) rosette-like structures (arrows). (RPE) Retinal pigment epithelium; (ONL) outer nuclear layer consisting of photoreceptor cells; (OPL) outer plexiform layer; (INL) inner nuclear layer; (L) lens. Magnification (C,E,G) 25×; (A,B,D,F,H) 200×.
Figure 3
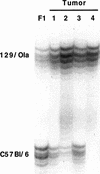
Retinoblastomas are derived from Rb−/−;p107 −/− cells. PCR products of a simple sequence repeat polymorphic for 129/Ola (190 nucleotides) and C57Bl/6 (160 nucleotides) in tumor DNAs. (Lane 1) Control liver of 129/Ola:C57Bl/6 F1 (50% 129/Ola). (Lane 2) Retinoblastoma in a Rb−/−;p107 −/− chimera (82% 129/Ola). (Lanes 3–5) Three retinoblastomas in Rb−/−; p107 −/−;hIRBP_p53DD_ chimeras (96%, 79%, and 99% 129/Ola, respectively). Percentages of 129/Ola contribution were determined using the PhosphorImager.
Figure 4
Retinoblastomas show immunocharacteristics of amacrine and glial cells. Sections of a normal retina (transgenic hIRBP_p53DD_ mouse, left parts) and a tumor-containing eye (chimeric Rb−/−;p107 −/−;hIRBP_p53DD_ mouse, right parts), immunostained with various antibodies using FITC, Texas Red, and DAB. (ONL) Outer nuclear layer containing photoreceptor (rod and cone) cells; (OPL) outer plexiform layer; (INL) inner nuclear layer containing horizontal, bipolar, Müller, and amacrine cells; (IPL) inner plexiform layer; (GCL) ganglion cell layer containing ganglion cells and astrocytes. (A) Extensive positive staining of the tumor with anti-syntaxin that labels amacrine cells in the INL and their synaptic processes in the IPL (FITC). (B) Whereas the control retina shows positive staining of the ONL and the outer half of the INL with anti-p53, the tumor is negative (DAB). Note the nuclear staining caused by the nuclear localization signal in p53DD. (C) No tumor staining with anti-IRBP that recognizes the outer segments of photoreceptor cells (FITC). (D) Tumor area with cells positive for anti-GFAP that labels glial cells (astrocytes in the GCL) and shows some positivity in the OPL (FITC). (E) Positive staining of the tumor with anti-NSE that immunoreacts with all neuronal cells (FITC). (F) No tumor staining with anti-NF200kd that labels the nerve fibers of ganglion cells in the GCL and axonless horizontal cells in the INL (FITC). (G–I) No double-stained (yellow) cells with anti-syntaxin (FITC) and anti-GFAP (Texas Red) in the tumor (H), or in the adjacent retina (I) showing a staining pattern suggestive of reactive Müller cells spanning across the retina. Dotted lines mark the border of the tumor (T). Magnification (A–G) 200×; (H,I) 600×.
Figure 5
Severe retinal abnormalities in chimeric Rb−/−;p107 −/− embryos. Histological sections of eyes, stained with hematoxilyn–eosin. (A) Normal retina of a chimeric Rb+/−;p107 −/− embryo (E17.5). (B) Dysplastic retina of a chimeric Rb−/−;p107 −/− embryo (E17.5), with rosettes (C), and an increased number of pyknotic nuclei (D) indicative of apoptosis. Magnification (A,B) 100×; (C) 200×; (D) 400×.
Figure 6
IRBP_-expressing Rb−/−;p107 −/− cells disappear from the retina before P15. Immunohistochemical staining for p53DD protein with pAb421 plus counterstaining with hematoxilyn. (A) No staining in the dysplastic retina of a chimeric Rb−/−;p107 −/− embryo (E17.5). (B) Retina of a chimeric Rb−/−;p107 −/−;hIRBP_p53DD embryo (E17.5) with positive cells in the ventricular layer (arrows). (C) No staining in the retina with malignant nodular growth of a young chimeric Rb−/−;p107 −/−;hIRBP_p53DD_ mouse (P15). (RPE) Retinal pigment epithelium; (VL) ventricular layer; (ONL) outer nuclear layer; (INL) inner nuclear layer. Magnification (A,B) 400×; (C) 200×.
Figure 7
Abnormal apoptosis during retinal development of chimeric Rb−/−;p107 −/− mice. FITC staining of apoptotic retinal cells (TUNEL assay). (A) Normal level of apoptosis in chimeric Rb+/−;p107 −/−;hIRBP_p53DD_ embryo (E17.5). (B) Increased level of apoptosis in chimeric Rb−/−;p107 −/− embryo (E17.5). (C) Normal level of apoptosis in wild-type C57Bl/6 neonate (P11). (D) Massive apoptosis in chimeric Rb−/−;p107 −/− neonate (P11). (ONL) Outer nuclear layer; (INL) inner nuclear layer; (L) lens. Magnification (A–D) 200×.
References
- Albert DM, Lahav M, Lesser R, Craft J. Recent observations regarding retinoblastoma, I: Ultrastructure, tissue culture growth, incidence, and animal models. Trans Ophthalmol Soc UK. 1974;94:909–928. - PubMed
- Al-Ubaidi MR, Font RL, Quiambao AB, Keener MJ, Liou GI, Overbeek PA, Baehr W. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J Cell Biol. 1992;119:1681–1687. - PMC - PubMed
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;20:286–290. - PubMed
- Beijersbergen RL, Carlée L, Kerkhoven RM, Bernards R. Regulation of the retinoblastoma protein-related p107 by G1 cyclin complexes. Genes & Dev. 1995;9:1340–1353. - PubMed
- Bernards R. E2F: A nodal point in cell cycle regulation. Biochim Biophys Acta. 1997;1333:33–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases