Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA - PubMed (original) (raw)
Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA
J B Ulmer et al. J Virol. 1998 Jul.
Abstract
DNA vaccination is an effective means of eliciting both humoral and cellular immunity, including cytotoxic T lymphocytes (CTL). Using an influenza virus model, we previously demonstrated that injection of DNA encoding influenza virus nucleoprotein (NP) induced major histocompatibility complex class I-restricted CTL and cross-strain protection from lethal virus challenge in mice (J. B. Ulmer et al., Science 259:1745-1749, 1993). In the present study, we have characterized in more detail the cellular immune responses induced by NP DNA, which included robust lymphoproliferation and Th1-type cytokine secretion (high levels of gamma interferon and interleukin-2 [IL-2], with little IL-4 or IL-10) in response to antigen-specific restimulation of splenocytes in vitro. These responses were mediated by CD4+ T cells, as shown by in vitro depletion of T-cell subsets. Taken together, these results indicate that immunization with NP DNA primes both cytolytic CD8+ T cells and cytokine-secreting CD4+ T cells. Further, we demonstrate by adoptive transfer and in vivo depletion of T-cell subsets that both of these types of T cells act as effectors in protective immunity against influenza virus challenge conferred by NP DNA.
Figures
FIG. 1
Lymphoproliferative responses after NP DNA vaccination. Female BALB/c mice were uninjected or injected with NP DNA (50 μg), control DNA (50 μg), or inactivated influenza virus (A/PR/8/34) (flu; 15 μg) on weeks 0 and 3 or were infected awake with 1,000 TCID50 of influenza virus (A/PR/8/34) on week 0. Spleens were collected and pooled from three mice per group on week 7 and restimulated in vitro with NP. Lymphoproliferation data are presented as a stimulation index.
FIG. 2
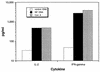
Cytokine secretion from restimulated spleen cells. Female BALB/c mice were injected with NP DNA (50 μg) or control DNA (50 μg) on weeks 0 and 3, and spleens were collected and pooled from three mice per group on week 7. Cells from DNA-injected mice were restimulated in vitro specifically with recombinant NP protein, and cells from NP DNA-injected mice were nonspecifically activated with the mitogen concanavalin A (Con A). Cytokines secreted into the culture supernatant were detected by ELISA and are presented as picograms/milliliter of culture supernatant.
FIG. 3
Anti-NP immunoglobulin subtype profile. Female BALB/c mice were injected with NP DNA (50 μg) or NP protein (10 μg) on weeks 0 and 3, and sera were collected on week 5. Anti-NP antibody subtypes were measured by ELISA as described in Materials and Methods. Data are presented as geometric mean ELISA titers ± standard errors of the means for groups of five mice.
FIG. 4
In vitro depletion of T-cell subsets. Female BALB/c mice were injected with NP DNA (200 μg) on weeks 0, 3, and 6, and spleens were collected and pooled from groups of three mice on week 23. T-cell subsets were prepared and restimulated as described in Materials and Methods. Cells from NP DNA-injected and uninjected mice were restimulated with NP protein and analyzed for proliferation plotted as a stimulation index (A) and secretion of IFN-γ (B) or IL-2 (C), as measured by ELISA and plotted as picograms/milliliter of culture supernatant.
FIG. 5
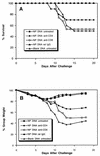
In vivo depletion of T-cell subsets. Groups of 10 female BALB/c mice were injected with NP DNA (200 μg) on weeks 0, 3, and 6 and then were untreated or treated with anti-CD4, anti-CD8, or control (rat IgG) antibody on week 9. As a negative control, mice were injected three times with control DNA (200 μg). All groups were challenged under anesthesia with 1,000 TCID50 of influenza virus A/HK/68 and monitored for survival (A) and weight loss (B). The results of two experiments were similar, and the data were combined in Fig. 5 to achieve an n of 20 per data point.
FIG. 6
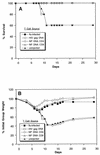
Adoptive transfer of T-cell subsets. Spleen cells from uninjected mice (solid triangles) or mice primed with influenza virus A/PR/8/34 (flu-infected; solid squares), immunized with NP DNA, or injected with HIV Gag DNA (open squares) were harvested. The NP DNA-primed spleen cells were enriched for CD4+ (open circles) or CD8+ T lymphocytes (open triangles). Spleen cells from mice immunized with HIV Gag DNA were restimulated with syngeneic cells pulsed with Gag peptide 193-212, while cells from the remaining groups were restimulated in vitro for 7 days with syngeneic cells infected with A/PR/8/34. These lymphocytes were adoptively transferred into age-matched naive mice (2.5 × 107 cells/mouse for the groups denoted by open and solid squares; 107 for groups denoted by open circles and triangles) that had been intranasally challenged with A/HK/68 (H3N2) 4 h previously. Data are plotted as percent survival (A) and weight loss (B) versus days after challenge for groups of 10 mice.
Similar articles
- Protective cellular immunity: cytotoxic T-lymphocyte responses against dominant and recessive epitopes of influenza virus nucleoprotein induced by DNA immunization.
Fu TM, Friedman A, Ulmer JB, Liu MA, Donnelly JJ. Fu TM, et al. J Virol. 1997 Apr;71(4):2715-21. doi: 10.1128/JVI.71.4.2715-2721.1997. J Virol. 1997. PMID: 9060624 Free PMC article. - The Role of Nucleoprotein in Immunity to Human Negative-Stranded RNA Viruses-Not Just Another Brick in the Viral Nucleocapsid.
Šantak M, Matić Z. Šantak M, et al. Viruses. 2022 Mar 3;14(3):521. doi: 10.3390/v14030521. Viruses. 2022. PMID: 35336928 Free PMC article. Review. - The Differentiation and Protective Function of Cytolytic CD4 T Cells in Influenza Infection.
Brown DM, Lampe AT, Workman AM. Brown DM, et al. Front Immunol. 2016 Mar 9;7:93. doi: 10.3389/fimmu.2016.00093. eCollection 2016. Front Immunol. 2016. PMID: 27014272 Free PMC article. Review.
Cited by
- Cooperativity between CD8+ T cells, non-neutralizing antibodies, and alveolar macrophages is important for heterosubtypic influenza virus immunity.
Laidlaw BJ, Decman V, Ali MA, Abt MC, Wolf AI, Monticelli LA, Mozdzanowska K, Angelosanto JM, Artis D, Erikson J, Wherry EJ. Laidlaw BJ, et al. PLoS Pathog. 2013 Mar;9(3):e1003207. doi: 10.1371/journal.ppat.1003207. Epub 2013 Mar 14. PLoS Pathog. 2013. PMID: 23516357 Free PMC article. - Characterization of humoral and cellular immune features of gamma-irradiated influenza vaccine.
Chen F, Seong Seo H, Ji HJ, Yang E, Choi JA, Yang JS, Song M, Han SH, Lim S, Lim JH, Ahn KB. Chen F, et al. Hum Vaccin Immunother. 2021 Feb 1;17(2):485-496. doi: 10.1080/21645515.2020.1780091. Epub 2020 Jul 9. Hum Vaccin Immunother. 2021. PMID: 32643515 Free PMC article. - Detection of Progeny Immune Responses after Intravenous Administration of DNA Vaccine to Pregnant Mice.
Xin KQ, Sasaki S, Kojima Y, Jounai N, Kumamoto Y, Hashimoto K, Shinoda K, Hamajima K, Okuda K. Xin KQ, et al. Biol Proced Online. 2002 Apr 23;3:91-101. doi: 10.1251/bpo27. Biol Proced Online. 2002. PMID: 12734575 Free PMC article. - Intranodal administration of mRNA encoding nucleoprotein provides cross-strain immunity against influenza in mice.
Joe PT, Christopoulou I, van Hoecke L, Schepens B, Ysenbaert T, Heirman C, Thielemans K, Saelens X, Aerts JL. Joe PT, et al. J Transl Med. 2019 Jul 25;17(1):242. doi: 10.1186/s12967-019-1991-3. J Transl Med. 2019. PMID: 31345237 Free PMC article. - Chlamydia trachomatis vaccine research through the years.
Schautteet K, De Clercq E, Vanrompay D. Schautteet K, et al. Infect Dis Obstet Gynecol. 2011;2011:963513. doi: 10.1155/2011/963513. Epub 2011 Jun 26. Infect Dis Obstet Gynecol. 2011. PMID: 21747646 Free PMC article. Review.
References
- Bender B S, Bell W E, Taylor S, Small P A., Jr Class I major histocompatibility complex-restricted cytotoxic T lymphocytes are not necessary for heterotypic immunity to influenza. J Infect Dis. 1994;170:1195–1200. - PubMed
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968;6:761–764. - PubMed
- Davis H L, Michel M L, Whalen R G. DNA-based immunization induces continuous secretion of hepatitis-b surface antigen and high levels of circulating antibody. Hum Mol Genet. 1993;2:1847–1851. - PubMed
- Deck R R, DeWitt C M, Donnelly J J, Liu M A, Ulmer J B. Characterization of humoral immune responses induced by an influenza hemagglutinin DNA vaccine. Vaccine. 1997;15:71–78. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous