Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 delta32 - PubMed (original) (raw)
doi: 10.1128/JVI.72.7.6040-6047.1998.
J A Nelson, V N KewalRamani, G Chang, S J O'Brien, J R Mascola, B Volsky, M Louder, G C White 2nd, D R Littman, R Swanstrom, T R O'Brien
Affiliations
- PMID: 9621067
- PMCID: PMC110409
- DOI: 10.1128/JVI.72.7.6040-6047.1998
Exclusive and persistent use of the entry coreceptor CXCR4 by human immunodeficiency virus type 1 from a subject homozygous for CCR5 delta32
N L Michael et al. J Virol. 1998 Jul.
Abstract
Individuals who are homozygous for the 32-bp deletion in the gene coding for the chemokine receptor and major human immunodeficiency virus type 1 (HIV-1) coreceptor CCR5 (CCR5 -/-) lack functional cell surface CCR5 molecules and are relatively resistant to HIV-1 infection. HIV-1 infection in CCR5 -/- individuals, although rare, has been increasingly documented. We now report that the viral quasispecies from one such individual throughout disease is homogenous, T cell line tropic, and phenotypically syncytium inducing (SI); exclusively uses CXCR4; and replicates well in CCR5 -/- primary T cells. The recently discovered coreceptors BOB and Bonzo are not used. Although early and persistent SI variants have been described in longitudinal studies, this is the first demonstration of exclusive and persistent CXCR4 usage. With the caveat that the earliest viruses available from this subject were from approximately 4 years following primary infection, these data suggest that HIV-1 infection can be mediated and persistently maintained by viruses which exclusively utilize CXCR4. The lack of evolution toward the available minor coreceptors in this subject underscores the dominant biological roles of the major coreceptors CCR5 and CXCR4. This and two similar subjects (R. Biti, R. Ffrench, J. Young, B. Bennetts, G. Stewart, and T. Liang, Nat. Med. 3:252-253, 1997; I. Theodoreu, L. Meyer, M. Magierowska, C. Katlama, and C. Rouzioux, Lancet 349:1219-1220, 1997) showed relatively rapid CD4+ T-cell declines despite average or low initial viral RNA load. Since viruses which use CXCR4 exclusively cannot infect macrophages, these data have implications for the relative infection of the T-cell compartment versus the macrophage compartment in vivo and for the development of CCR5-based therapeutics.
Figures
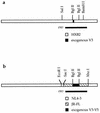
FIG. 1
Construction of isogenic recombinant proviruses. (a) Exogenous V3 region env sequences were inserted into a portion of the HIV-1HXB2 env gene contained within the _Sal_I-_Bam_HI fragment and then inserted into the HIV-1HXB2 proviral background. (b) Exogenous sequences V3 through V5 contained by _Bgl_II sites were inserted into the HIV-1JR-FL background bounded by _Sac_I and _Xho_I sites and then inserted into the HIV-1NL4-3 proviral background.
FIG. 2
Analysis by V3-HTA. V3 loop RT-PCR products from the June 1986 serum sample from patient UNC116 (lane 86) and control YU-2 and NL4-3 recombinant proviral clones were annealed with the JR-FL V3 probe that was 35S labeled on one strand. The heteroduplexes were resolved on a native polyacrylamide gel and visualized by autoradiography. Only the bottom half of the autoradiograph is shown. The positions of the single-stranded probe (upper arrow) and double-stranded probe homoduplexes (lower arrow) are indicated. Heteroduplexes in each lane resolved between the single-stranded probe and probe homoduplexes.
FIG. 3
HIV-1 entry coreceptor typing of viral stocks in GHOST cells. HOS cells nonpermissive for HIV-1 infections were transduced with human CD4 and the GFP under the control of the HIV-2 LTR to generate parental GHOST cells. These cells were then individually transduced with the HIV-1 entry coreceptor cDNAs indicated at the top. HIV-1 entry and subsequent tat gene expression transactivates the HIV-2 LTR and upregulates the expression of GFP. Following infection of parental and coreceptor-transduced cells with equivalent amounts of specific viral stocks (indicated at the left), the cells were fixed and analyzed by flow cytometry. For each fluorescence-activated cell sorter profile, linear forward side scatter is plotted against log fluorescence intensity for GFP in the FL1 window. Basal-level production of GFP is red; transactivated GFP expression is green.
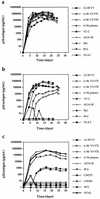
FIG. 4
Replication kinetics of viral stocks in CCR5 +/+ (a) and CCR5 −/− (b) PBMC and CCR5 +/+ macrophages (c). One hundred and 500 TCID50 of PBMC-derived viral stocks were used to infect PHA- and IL-2-stimulated PBMC and monocyte-derived macrophages, respectively. Virus replication was monitored by supernatant p24 antigen production.
Similar articles
- Chemokine coreceptor usage by diverse primary isolates of human immunodeficiency virus type 1.
Zhang L, He T, Huang Y, Chen Z, Guo Y, Wu S, Kunstman KJ, Brown RC, Phair JP, Neumann AU, Ho DD, Wolinsky SM. Zhang L, et al. J Virol. 1998 Nov;72(11):9307-12. doi: 10.1128/JVI.72.11.9307-9312.1998. J Virol. 1998. PMID: 9765480 Free PMC article. - Role of CXCR4 in cell-cell fusion and infection of monocyte-derived macrophages by primary human immunodeficiency virus type 1 (HIV-1) strains: two distinct mechanisms of HIV-1 dual tropism.
Yi Y, Isaacs SN, Williams DA, Frank I, Schols D, De Clercq E, Kolson DL, Collman RG. Yi Y, et al. J Virol. 1999 Sep;73(9):7117-25. doi: 10.1128/JVI.73.9.7117-7125.1999. J Virol. 1999. PMID: 10438797 Free PMC article. - Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype.
Björndal A, Deng H, Jansson M, Fiore JR, Colognesi C, Karlsson A, Albert J, Scarlatti G, Littman DR, Fenyö EM. Björndal A, et al. J Virol. 1997 Oct;71(10):7478-87. doi: 10.1128/JVI.71.10.7478-7487.1997. J Virol. 1997. PMID: 9311827 Free PMC article. - HIV-1 infection in individuals with the CCR5-Delta32/Delta32 genotype: acquisition of syncytium-inducing virus at seroconversion.
Sheppard HW, Celum C, Michael NL, O'Brien S, Dean M, Carrington M, Dondero D, Buchbinder SP. Sheppard HW, et al. J Acquir Immune Defic Syndr. 2002 Mar 1;29(3):307-13. doi: 10.1097/00126334-200203010-00013. J Acquir Immune Defic Syndr. 2002. PMID: 11873082 Review. - CCR5 as a Coreceptor for Human Immunodeficiency Virus and Simian Immunodeficiency Viruses: A Prototypic Love-Hate Affair.
Jasinska AJ, Pandrea I, Apetrei C. Jasinska AJ, et al. Front Immunol. 2022 Jan 27;13:835994. doi: 10.3389/fimmu.2022.835994. eCollection 2022. Front Immunol. 2022. PMID: 35154162 Free PMC article. Review.
Cited by
- Inhibition of human immunodeficiency virus replication by a dual CCR5/CXCR4 antagonist.
Princen K, Hatse S, Vermeire K, Aquaro S, De Clercq E, Gerlach LO, Rosenkilde M, Schwartz TW, Skerlj R, Bridger G, Schols D. Princen K, et al. J Virol. 2004 Dec;78(23):12996-3006. doi: 10.1128/JVI.78.23.12996-13006.2004. J Virol. 2004. PMID: 15542651 Free PMC article. - Transmission of highly virulent CXCR4 tropic HIV-1 through the mucosal route in an individual with a wild-type CCR5 genotype.
Song H, Marichannegowda M, Setua S, Bose M, Sanders-Buell E, King D, Zemil M, Wieczorek L, Diaz-Mendez F, Chomont N, Thomas R, Francisco L, Eller LA, Polonis V, Tovanabutra S, Tagaya Y, Michael N, Robb M. Song H, et al. Res Sq [Preprint]. 2023 Sep 25:rs.3.rs-3359209. doi: 10.21203/rs.3.rs-3359209/v1. Res Sq. 2023. PMID: 37841838 Free PMC article. Updated. Preprint. - The biology of CCR5 and CXCR4.
Alkhatib G. Alkhatib G. Curr Opin HIV AIDS. 2009 Mar;4(2):96-103. doi: 10.1097/COH.0b013e328324bbec. Curr Opin HIV AIDS. 2009. PMID: 19339947 Free PMC article. Review. - Genetic factors for aids progression: Analyses of HIV-1 resistant genes in India and therapeutic potentials of novel catalytic RNA and DNA molecules.
Chakraborti S, Banerjea AC. Chakraborti S, et al. Indian J Clin Biochem. 2002 Jul;17(2):91-5. doi: 10.1007/BF02867978. Indian J Clin Biochem. 2002. PMID: 23105357 Free PMC article. No abstract available. - Loss of naïve cells accompanies memory CD4+ T-cell depletion during long-term progression to AIDS in Simian immunodeficiency virus-infected macaques.
Nishimura Y, Igarashi T, Buckler-White A, Buckler C, Imamichi H, Goeken RM, Lee WR, Lafont BA, Byrum R, Lane HC, Hirsch VM, Martin MA. Nishimura Y, et al. J Virol. 2007 Jan;81(2):893-902. doi: 10.1128/JVI.01635-06. Epub 2006 Nov 8. J Virol. 2007. PMID: 17093193 Free PMC article.
References
- Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
- Alkhatib G, Liao F, Berger E A, Farber J M, Peden K W C. A new SIV co-receptor, STRL33. Nature. 1997;388:238. - PubMed
- Atchison R E, Gosling J, Monteclaro F S, Franci C, Digilio L, Charo I F, Goldsmith M A. Multiple extracellular elements of CCR5 and HIV-1 entry: dissociation from response to chemokines. Science. 1996;274:1924–1926. - PubMed
- Balotta C, Bagnarelli P, Violin M, Ridolfo A L, Zhou D, Berlusconi A, Corvasce S, Corbellino M, Clementi M, Clerici M, Moroni M, Galli M. Homozygous Δ32 deletion of the CCR-5 chemokine receptor gene in an HIV-1-infected patient. AIDS. 1997;11:F67–F71. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials