Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP) - PubMed (original) (raw)
Nuclear factor of activated T cells (NFAT)-dependent transactivation regulated by the coactivators p300/CREB-binding protein (CBP)
C García-Rodríguez et al. J Exp Med. 1998.
Abstract
p300 and cAMP response element-binding protein (CREB)-binding protein (CBP) are members of a family of coactivators involved in the regulation of transcription and chromatin. We show that transcription factors of the nuclear factor of activated T cells (NFAT) family bind p300/CBP and recruit histone acetyltransferase activity from T cell nuclear extracts. The NH2-terminal transactivation domain of NFAT1 and the phospho-CREB- and E1A-binding sites of p300/CBP are involved in the interaction. The viral oncoprotein E1A inhibits NFAT-dependent transactivation in a p300-dependent manner. Recruitment of the coactivators p300/CBP by the transactivation domains of NFAT proteins is likely to play a critical role in NFAT-dependent gene expression during the immune response.
Figures
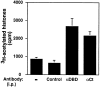
Figure 1
NFAT1 associates with HATs in nuclear extracts. Cl.7W2 T cells were stimulated with ionomycin and PMA for 25 min at 37°C, and nuclear extracts were immunoprecipitated (i.p.) with antibodies against the DBD (α_DBD_) or a COOH-terminal peptide (α_Ct_) of NFAT1. An irrelevant antibody was used as a control. The immune complexes were tested for HAT activity as described in Materials and Methods. The results are representative of three independent experiments. Bars, The mean and range of duplicates in one such experiment.
Figure 2
Interaction of p300 and NFAT1 in cells. (A) 293T cells expressing NFAT1 and HA-p300 were stimulated with ionomycin and PMA for 25 min at 37°C. Whole-cell extracts were immunoprecipitated (i.p.) with antibodies against an NH2-terminal peptide (α_Nt_), the DBD (α_DBD_), and a COOH-terminal peptide (α_Ct_) of NFAT1. An irrelevant antibody was used as a control. The immune complexes were analyzed by Western blotting for HA-p300 (top) and NFAT1 (bottom, blot with α_Nt_). The expressed HA-p300, detected with anti-HA (α_HA_), appeared as several bands (lanes 5 and 6), and the bottom band seemed to be preferentially coimmunoprecipitated with NFAT1 (lanes 3 and 4). (B) Lysates from 293T cells expressing NFAT1 alone, HA-p300 alone, or both were immunoprecipitated (i.p.) with anti-HA (α_HA_), and immune complexes were analyzed by Western blotting for HA-p300 (top) and NFAT1 (bottom). Input, 1% of the total amount of cell extract used for immunoprecipitations. Arrows, The different forms detected by immunoblotting.
Figure 2
Interaction of p300 and NFAT1 in cells. (A) 293T cells expressing NFAT1 and HA-p300 were stimulated with ionomycin and PMA for 25 min at 37°C. Whole-cell extracts were immunoprecipitated (i.p.) with antibodies against an NH2-terminal peptide (α_Nt_), the DBD (α_DBD_), and a COOH-terminal peptide (α_Ct_) of NFAT1. An irrelevant antibody was used as a control. The immune complexes were analyzed by Western blotting for HA-p300 (top) and NFAT1 (bottom, blot with α_Nt_). The expressed HA-p300, detected with anti-HA (α_HA_), appeared as several bands (lanes 5 and 6), and the bottom band seemed to be preferentially coimmunoprecipitated with NFAT1 (lanes 3 and 4). (B) Lysates from 293T cells expressing NFAT1 alone, HA-p300 alone, or both were immunoprecipitated (i.p.) with anti-HA (α_HA_), and immune complexes were analyzed by Western blotting for HA-p300 (top) and NFAT1 (bottom). Input, 1% of the total amount of cell extract used for immunoprecipitations. Arrows, The different forms detected by immunoblotting.
Figure 3
Regions of NFAT1 and CBP involved in the interaction. (A) In vitro binding experiments were performed using 6xHisNFAT1(1–415) and GST-CBP fusion proteins immobilized on glutathione-Sepharose beads. After six washes, the samples were analyzed by Western blotting analysis for NFAT1. (B) 293T cells coexpressing HA-p300 and either full-length NFAT1 (NFAT1-FL) or NFAT1ΔReg were stimulated with ionomycin and PMA for 25 min at 37°C. Whole-cell extracts were immunoprecipitated with anti-HA (α_HA_). Immune complexes were analyzed by Western blotting for HA-p300 (top) and NFAT1 (bottom). Input, 1% of the total amount of cell extract used for immunoprecipitations. Arrows, The different forms detected by immunoblotting.
Figure 3
Regions of NFAT1 and CBP involved in the interaction. (A) In vitro binding experiments were performed using 6xHisNFAT1(1–415) and GST-CBP fusion proteins immobilized on glutathione-Sepharose beads. After six washes, the samples were analyzed by Western blotting analysis for NFAT1. (B) 293T cells coexpressing HA-p300 and either full-length NFAT1 (NFAT1-FL) or NFAT1ΔReg were stimulated with ionomycin and PMA for 25 min at 37°C. Whole-cell extracts were immunoprecipitated with anti-HA (α_HA_). Immune complexes were analyzed by Western blotting for HA-p300 (top) and NFAT1 (bottom). Input, 1% of the total amount of cell extract used for immunoprecipitations. Arrows, The different forms detected by immunoblotting.
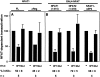
Figure 4
E1A inhibits NFAT-dependent transactivation in a p300-dependent manner. (A) The NFAT3x-Luc reporter plasmid and expression plasmids encoding full-length NFAT1 (NFAT1-FL) or NFAT1ΔReg were cotransfected into Jurkat cells along with expression plasmids encoding wild-type E1A (WT) or the E1A mutant defective for p300 binding (Mut). (B) The GAL4-Luc reporter and expression plasmid encoding GAL4-NFAT1(1–415), GAL4-NFAT2(1–418), or GAL4-NFAT1ΔSP2 were cotransfected into Jurkat cells along with expression plasmids encoding wild-type E1A (WT) or the E1A mutant defective in p300 binding (Mut). After 36 h of transfection, cells were left unstimulated or stimulated overnight with ionomycin and PMA and assayed for luciferase assay. Results are representative of several experiments. Transfection efficiencies were normalized by measuring hGH expression from a cotransfected RSV-hGH plasmid.
Similar articles
- A role for CREB binding protein and p300 transcriptional coactivators in Ets-1 transactivation functions.
Yang C, Shapiro LH, Rivera M, Kumar A, Brindle PK. Yang C, et al. Mol Cell Biol. 1998 Apr;18(4):2218-29. doi: 10.1128/MCB.18.4.2218. Mol Cell Biol. 1998. PMID: 9528793 Free PMC article. - Epstein-Barr virus nuclear protein 2 interacts with p300, CBP, and PCAF histone acetyltransferases in activation of the LMP1 promoter.
Wang L, Grossman SR, Kieff E. Wang L, et al. Proc Natl Acad Sci U S A. 2000 Jan 4;97(1):430-5. doi: 10.1073/pnas.97.1.430. Proc Natl Acad Sci U S A. 2000. PMID: 10618435 Free PMC article. - CBP and p300: HATs for different occasions.
Kalkhoven E. Kalkhoven E. Biochem Pharmacol. 2004 Sep 15;68(6):1145-55. doi: 10.1016/j.bcp.2004.03.045. Biochem Pharmacol. 2004. PMID: 15313412 Review. - Versatile molecular glue. Transcriptional control.
Janknecht R, Hunter T. Janknecht R, et al. Curr Biol. 1996 Aug 1;6(8):951-4. doi: 10.1016/s0960-9822(02)00636-x. Curr Biol. 1996. PMID: 8805328 Review.
Cited by
- SIRT7 activates quiescent hair follicle stem cells to ensure hair growth in mice.
Li G, Tang X, Zhang S, Jin M, Wang M, Deng Z, Liu Z, Qian M, Shi W, Wang Z, Xie H, Li J, Liu B. Li G, et al. EMBO J. 2020 Sep 15;39(18):e104365. doi: 10.15252/embj.2019104365. Epub 2020 Jul 21. EMBO J. 2020. PMID: 32696520 Free PMC article. - Role of PARP Inhibitors in Cancer Immunotherapy: Potential Friends to Immune Activating Molecules and Foes to Immune Checkpoints.
Franzese O, Graziani G. Franzese O, et al. Cancers (Basel). 2022 Nov 16;14(22):5633. doi: 10.3390/cancers14225633. Cancers (Basel). 2022. PMID: 36428727 Free PMC article. Review. - Human T lymphotropic virus type 1 accessory protein p12I modulates calcium-mediated cellular gene expression and enhances p300 expression in T lymphocytes.
Nair A, Michael B, Hiraragi H, Fernandez S, Feuer G, Boris-Lawrie K, Lairmore M. Nair A, et al. AIDS Res Hum Retroviruses. 2005 Apr;21(4):273-84. doi: 10.1089/aid.2005.21.273. AIDS Res Hum Retroviruses. 2005. PMID: 15943569 Free PMC article. - HIV eradication: combinatorial approaches to activate latent viruses.
De Crignis E, Mahmoudi T. De Crignis E, et al. Viruses. 2014 Nov 21;6(11):4581-608. doi: 10.3390/v6114581. Viruses. 2014. PMID: 25421889 Free PMC article. Review. - The effects of cocaine on HIV transcription.
Tyagi M, Weber J, Bukrinsky M, Simon GL. Tyagi M, et al. J Neurovirol. 2016 Jun;22(3):261-74. doi: 10.1007/s13365-015-0398-z. Epub 2015 Nov 16. J Neurovirol. 2016. PMID: 26572787 Free PMC article. Review.
References
- Brownell JE, Allis D. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev. 1996;6:176–184. - PubMed
- Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. - PubMed
- Wu C. Chromatin remodeling and the control of gene expression. J Biol Chem. 1997;272:28171–28174. - PubMed
- Ogryzko VV, Schiltz RL, Russanova V, Howard BH, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. - PubMed
- Bannister AJ, Kourazides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous