Interaction between mitochondria and the actin cytoskeleton in budding yeast requires two integral mitochondrial outer membrane proteins, Mmm1p and Mdm10p - PubMed (original) (raw)
Interaction between mitochondria and the actin cytoskeleton in budding yeast requires two integral mitochondrial outer membrane proteins, Mmm1p and Mdm10p
I Boldogh et al. J Cell Biol. 1998.
Abstract
Transfer of mitochondria to daughter cells during yeast cell division is essential for viable progeny. The actin cytoskeleton is required for this process, potentially as a track to direct mitochondrial movement into the bud. Sedimentation assays reveal two different components required for mitochondria-actin interactions: (1) mitochondrial actin binding protein(s) (mABP), a peripheral mitochondrial outer membrane protein(s) with ATP-sensitive actin binding activity, and (2) a salt-inextractable, presumably integral, membrane protein(s) required for docking of mABP on the organelle. mABP activity is abolished by treatment of mitochondria with high salt. Addition of either the salt-extracted mitochondrial peripheral membrane proteins (SE), or a protein fraction with ATP-sensitive actin-binding activity isolated from SE, to salt-washed mitochondria restores this activity. mABP docking activity is saturable, resistant to high salt, and inhibited by pre-treatment of salt-washed mitochondria with papain. Two integral mitochondrial outer membrane proteins, Mmm1p (Burgess, S.M., M. Delannoy, and R.E. Jensen. 1994. J.Cell Biol. 126:1375-1391) and Mdm10p, (Sogo, L.F., and M.P. Yaffe. 1994. J.Cell Biol. 126:1361- 1373) are required for these actin-mitochondria interactions. Mitochondria isolated from an mmm1-1 temperature-sensitive mutant or from an mdm10 deletion mutant show no mABP activity and no mABP docking activity. Consistent with this, mitochondrial motility in vivo in mmm1-1 and mdm10Delta mutants appears to be actin independent. Depolymerization of F-actin using latrunculin-A results in loss of long-distance, linear movement and a fivefold decrease in the velocity of mitochondrial movement. Mitochondrial motility in mmm1-1 and mdm10Delta mutants is indistinguishable from that in latrunculin-A-treated wild-type cells. We propose that Mmm1p and Mdm10p are required for docking of mABP on the surface of yeast mitochondria and coupling the organelle to the actin cytoskeleton.
Figures
Figure 1
Mitochondrial morphology is defective after short-term treatment with latrunculin-A. Wild-type cells expressing CS1-GFP were grown at 30°C in a synthetic galactose-containing media lacking uracil. 0.25 mM latrunculin-A dissolved in DMSO (C–D) or equal volume of DMSO alone (A–B) was added to mid-log phase cultures. After a 20-min incubation cells were fixed and stained with rhodamine phalloidin to visualize actin structures (B and D). Mitochondria are labeled with GFP (A and C). Bar, 1 μm.
Figure 2
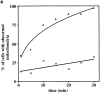
Lat-A treatment results in loss of linear, long-distance mitochondrial movement. (a) Cells containing GFP-labeled mitochondria were incubated in the presence (▵) or absence (○) of 0.25 mM Lat-A, and mitochondrial morphology and position were evaluated as a function of time of incubation. Cells containing fragmented mitochondria, spherical mitochondria, or mitochondria that did not align along the mother–bud axis were scored as abnormal (n > 100 for each time point). (b) Paths of GFP-labeled mitochondria were obtained from cells incubated in the absence (A) or presence (B) of 0.25 mM Lat-A for 20 min. Images were acquired at 20-s intervals over a period of 10 min. Tracings of the movements of individual mitochondria were made by marking the tip of motile organelles during the time in which they remained in the plane of focus. The points denote the position of organelles at 20-s intervals. Tracings are shown relative to the boundary of the dividing yeast cell. Bar, 1 μm.
Figure 2
Lat-A treatment results in loss of linear, long-distance mitochondrial movement. (a) Cells containing GFP-labeled mitochondria were incubated in the presence (▵) or absence (○) of 0.25 mM Lat-A, and mitochondrial morphology and position were evaluated as a function of time of incubation. Cells containing fragmented mitochondria, spherical mitochondria, or mitochondria that did not align along the mother–bud axis were scored as abnormal (n > 100 for each time point). (b) Paths of GFP-labeled mitochondria were obtained from cells incubated in the absence (A) or presence (B) of 0.25 mM Lat-A for 20 min. Images were acquired at 20-s intervals over a period of 10 min. Tracings of the movements of individual mitochondria were made by marking the tip of motile organelles during the time in which they remained in the plane of focus. The points denote the position of organelles at 20-s intervals. Tracings are shown relative to the boundary of the dividing yeast cell. Bar, 1 μm.
Figure 3
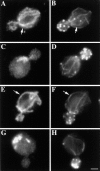
The organization of mitochondria and actin in mmm1-1 and mdm10Δ strains. MMM1 parent (YPH252; A–B) and mmm1-1 mutant (YPH253; C–D) cells were grown overnight at 22°C, and then shifted to 37°C for 3 h. MDM10 parent (MYY291; E–F) and mdm10Δ mutant (MYY504; G–H) cells were grown at 22°C overnight. Fixed cells were stained for mitochondria by indirect immunofluorescence using an antibody raised against mitochondrial outer membrane proteins (A, C, E, and G) and for actin using rhodamine phalloidin (B, D, F, and H). Arrows point to examples of alignment of mitochondria with actin cables in wild-type cells. Bar, 1 μm.
Figure 4
Mitochondrial motility is defective in mmm1 and mdm10 mutants. Tracings of mitochondrial movement in MMM1 (A), mmm1-1 (B), MDM10 (C), and mdm10Δ cells (D) were performed as for Fig. 2. The points denote the position of organelles at 20-s intervals. Tracings are shown relative to the boundary of the dividing yeast cell. Bar, 1 μm.
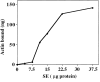
Figure 5
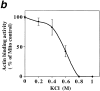
ATP-sensitive actin-binding activity of mitochondria requires a peripheral membrane protein which binds reversibly to mitochondria. (a) Sedimentation assays of isolated mitochondria from the D273-10B strain with phalloidin-stabilized F-actin in the presence or absence of ATP were carried out as described in Materials and Methods. The mitochondrial pellets of the sedimented reaction mixtures were evaluated for the levels of actin and of two mitochondrial marker proteins (cytochrome b2 and porin) by Western blot analysis. (1 and 2) Untreated (control) mitochondria (M); (3 and 4) salt-washed mitochondria (SW); and (5 and 6) salt-washed mitochondria (SW) pretreated with mitochondrial salt extract (SE). (b) The effect of KCl concentration on isolating SE. Isolated mitochondria were incubated in increasing amounts of salt. KCl was removed and the level of ATP-sensitive actin-binding activity of SW was tested using the sedimentation assay. The relative ATP-sensitive actin binding is shown here. 100% was defined as the amount of actin-binding activity in control, untreated mitochondria.
Figure 5
ATP-sensitive actin-binding activity of mitochondria requires a peripheral membrane protein which binds reversibly to mitochondria. (a) Sedimentation assays of isolated mitochondria from the D273-10B strain with phalloidin-stabilized F-actin in the presence or absence of ATP were carried out as described in Materials and Methods. The mitochondrial pellets of the sedimented reaction mixtures were evaluated for the levels of actin and of two mitochondrial marker proteins (cytochrome b2 and porin) by Western blot analysis. (1 and 2) Untreated (control) mitochondria (M); (3 and 4) salt-washed mitochondria (SW); and (5 and 6) salt-washed mitochondria (SW) pretreated with mitochondrial salt extract (SE). (b) The effect of KCl concentration on isolating SE. Isolated mitochondria were incubated in increasing amounts of salt. KCl was removed and the level of ATP-sensitive actin-binding activity of SW was tested using the sedimentation assay. The relative ATP-sensitive actin binding is shown here. 100% was defined as the amount of actin-binding activity in control, untreated mitochondria.
Figure 6
mABP is a peripheral mitochondrial membrane protein. Fractions recovered from F-actin affinity chromatography of SE (see Materials and Methods) were incubated with SW for 10 min at 4°C. mABP activity in these organelles was then assayed as for Fig. 5. Mito, control mitochondria; SW, salt-washed mitochondria; FT, flow through from the actin affinity column; W, eluate after washing the column with 0.2 M KCl.
Figure 7
The effect of increasing amount of SE on restoration of mABP activity in SW. SW were pre-treated with increasing amounts of salt-extractable membrane proteins (SE). Mitochondria were re-isolated and their ATP-sensitive binding activity was determined by sedimentation assay. The level of actin cosedimenting with mitochondria was measured by comparing the band densities of organelle-associated actin with those of known quantities of actin.
Figure 8
Salt-inextractable mitochondrial membrane protein(s) are required for binding of mABP to mitochondria. To confirm that mABP is a protein, salt-extracted mitochondrial proteins (SE) were treated with trypsin and chymotrypsin (protease SE). The ability of protease-treated SE to restore F-actin–binding activity to SW was determined as for Fig. 5. To confirm that mABP docking is protein mediated, salt-washed mitochondria (SW) were incubated with papain (protease SW). Protease-treated mitochondria were recovered by centrifugation at 10,000 g for 5 min at 4°C. Actin-binding activity of each sample was determined as in Fig. 5, and the relative ATP-sensitive actin-binding activities are presented. 100% was defined as the amount of binding activity in the control mitochondria sample. In all cases, the recovery of actin with the mitochondrial pellet was normalized for equal recovery of porin.
Figure 9
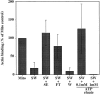
mmm1-1 and mdm10Δ mitochondria, but not om45Δ mitochondria, are defective in ATP-sensitive actin-binding activity. Mitochondria were isolated from wild-type parent and the corresponding mutant strains: MMM1, mmm1-1 (a), MDM10, mdm10Δ (b), and OM45, om45Δ (c). Sedimentation assays of isolated mitochondria were carried out with phalloidin-stabilized F-actin in the presence and absence of ATP (+/− ATP) at 30°C and also at 23°C in the case of MMM1 and mmm1-1 mitochondria. The mitochondrial pellets of the sedimented reaction mixtures were evaluated for the levels of actin, cytochrome b2, and porin by Western blot analysis. M, untreated mitochondria incubated with F-actin. SW, salt-washed mitochondria incubated with F-actin. SW+SE, salt-washed mitochondria from the strains indicated pretreated with the corresponding wild-type mitochondrial salt extract before incubation with F-actin.
Figure 9
mmm1-1 and mdm10Δ mitochondria, but not om45Δ mitochondria, are defective in ATP-sensitive actin-binding activity. Mitochondria were isolated from wild-type parent and the corresponding mutant strains: MMM1, mmm1-1 (a), MDM10, mdm10Δ (b), and OM45, om45Δ (c). Sedimentation assays of isolated mitochondria were carried out with phalloidin-stabilized F-actin in the presence and absence of ATP (+/− ATP) at 30°C and also at 23°C in the case of MMM1 and mmm1-1 mitochondria. The mitochondrial pellets of the sedimented reaction mixtures were evaluated for the levels of actin, cytochrome b2, and porin by Western blot analysis. M, untreated mitochondria incubated with F-actin. SW, salt-washed mitochondria incubated with F-actin. SW+SE, salt-washed mitochondria from the strains indicated pretreated with the corresponding wild-type mitochondrial salt extract before incubation with F-actin.
Figure 9
mmm1-1 and mdm10Δ mitochondria, but not om45Δ mitochondria, are defective in ATP-sensitive actin-binding activity. Mitochondria were isolated from wild-type parent and the corresponding mutant strains: MMM1, mmm1-1 (a), MDM10, mdm10Δ (b), and OM45, om45Δ (c). Sedimentation assays of isolated mitochondria were carried out with phalloidin-stabilized F-actin in the presence and absence of ATP (+/− ATP) at 30°C and also at 23°C in the case of MMM1 and mmm1-1 mitochondria. The mitochondrial pellets of the sedimented reaction mixtures were evaluated for the levels of actin, cytochrome b2, and porin by Western blot analysis. M, untreated mitochondria incubated with F-actin. SW, salt-washed mitochondria incubated with F-actin. SW+SE, salt-washed mitochondria from the strains indicated pretreated with the corresponding wild-type mitochondrial salt extract before incubation with F-actin.
Similar articles
- A protein complex containing Mdm10p, Mdm12p, and Mmm1p links mitochondrial membranes and DNA to the cytoskeleton-based segregation machinery.
Boldogh IR, Nowakowski DW, Yang HC, Chung H, Karmon S, Royes P, Pon LA. Boldogh IR, et al. Mol Biol Cell. 2003 Nov;14(11):4618-27. doi: 10.1091/mbc.e03-04-0225. Epub 2003 Sep 17. Mol Biol Cell. 2003. PMID: 13679517 Free PMC article. - Mmm1p, a mitochondrial outer membrane protein, is connected to mitochondrial DNA (mtDNA) nucleoids and required for mtDNA stability.
Hobbs AE, Srinivasan M, McCaffery JM, Jensen RE. Hobbs AE, et al. J Cell Biol. 2001 Jan 22;152(2):401-10. doi: 10.1083/jcb.152.2.401. J Cell Biol. 2001. PMID: 11266455 Free PMC article. - Rsp5p, a new link between the actin cytoskeleton and endocytosis in the yeast Saccharomyces cerevisiae.
Kamińska J, Gajewska B, Hopper AK, Zoładek T. Kamińska J, et al. Mol Cell Biol. 2002 Oct;22(20):6946-8. doi: 10.1128/MCB.22.20.6946-6958.2002. Mol Cell Biol. 2002. PMID: 12242276 Free PMC article. - Interactions of mitochondria with the actin cytoskeleton.
Boldogh IR, Pon LA. Boldogh IR, et al. Biochim Biophys Acta. 2006 May-Jun;1763(5-6):450-62. doi: 10.1016/j.bbamcr.2006.02.014. Epub 2006 Mar 29. Biochim Biophys Acta. 2006. PMID: 16624426 Review. - Mitochondrial manoeuvres: latest insights and hypotheses on mitochondrial partitioning during mitosis in Saccharomyces cerevisiae.
Peraza-Reyes L, Crider DG, Pon LA. Peraza-Reyes L, et al. Bioessays. 2010 Dec;32(12):1040-9. doi: 10.1002/bies.201000083. Epub 2010 Sep 30. Bioessays. 2010. PMID: 20886527 Review.
Cited by
- ER-mitochondria contacts promote mitochondrial-derived compartment biogenesis.
English AM, Schuler MH, Xiao T, Kornmann B, Shaw JM, Hughes AL. English AM, et al. J Cell Biol. 2020 Dec 7;219(12):e202002144. doi: 10.1083/jcb.202002144. J Cell Biol. 2020. PMID: 33090183 Free PMC article. - The Golgi alpha-1,6 mannosyltransferase KlOch1p of Kluyveromyces lactis is required for Ca2+/calmodulin-based signaling and for proper mitochondrial functionality.
Zanni E, Farina F, Ricci A, Mancini P, Frank C, Palleschi C, Uccelletti D. Zanni E, et al. BMC Cell Biol. 2009 Dec 14;10:86. doi: 10.1186/1471-2121-10-86. BMC Cell Biol. 2009. PMID: 20003441 Free PMC article. - Moving mitochondria: establishing distribution of an essential organelle.
Frederick RL, Shaw JM. Frederick RL, et al. Traffic. 2007 Dec;8(12):1668-1675. doi: 10.1111/j.1600-0854.2007.00644.x. Epub 2007 Oct 17. Traffic. 2007. PMID: 17944806 Free PMC article. Review. - Maintenance and integrity of the mitochondrial genome: a plethora of nuclear genes in the budding yeast.
Contamine V, Picard M. Contamine V, et al. Microbiol Mol Biol Rev. 2000 Jun;64(2):281-315. doi: 10.1128/MMBR.64.2.281-315.2000. Microbiol Mol Biol Rev. 2000. PMID: 10839818 Free PMC article. Review. - Importance of mitochondrial dynamics during meiosis and sporulation.
Gorsich SW, Shaw JM. Gorsich SW, et al. Mol Biol Cell. 2004 Oct;15(10):4369-81. doi: 10.1091/mbc.e03-12-0875. Epub 2004 Jul 14. Mol Biol Cell. 2004. PMID: 15254264 Free PMC article.
References
- Baker D, Schekman R. Reconstitution of protein transport using broken yeast spheroplasts. Methods Cell Biol. 1989;31:127–141. - PubMed
- Bradley TJ, Satir P. Evidence of microfilament-associated mitochondrial movement. J Supramol Struct. 1979;12:165–175. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases