Differential nuclear translocation and transactivation potential of beta-catenin and plakoglobin - PubMed (original) (raw)
Comparative Study
Differential nuclear translocation and transactivation potential of beta-catenin and plakoglobin
I Simcha et al. J Cell Biol. 1998.
Abstract
beta-Catenin and plakoglobin are homologous proteins that function in cell adhesion by linking cadherins to the cytoskeleton and in signaling by transactivation together with lymphoid-enhancing binding/T cell (LEF/TCF) transcription factors. Here we compared the nuclear translocation and transactivation abilities of beta-catenin and plakoglobin in mammalian cells. Overexpression of each of the two proteins in MDCK cells resulted in nuclear translocation and formation of nuclear aggregates. The beta-catenin-containing nuclear structures also contained LEF-1 and vinculin, while plakoglobin was inefficient in recruiting these molecules, suggesting that its interaction with LEF-1 and vinculin is significantly weaker. Moreover, transfection of LEF-1 translocated endogenous beta-catenin, but not plakoglobin to the nucleus. Chimeras consisting of Gal4 DNA-binding domain and the transactivation domains of either plakoglobin or beta-catenin were equally potent in transactivating a Gal4-responsive reporter, whereas activation of LEF-1- responsive transcription was significantly higher with beta-catenin. Overexpression of wild-type plakoglobin or mutant beta-catenin lacking the transactivation domain induced accumulation of the endogenous beta-catenin in the nucleus and LEF-1-responsive transactivation. It is further shown that the constitutive beta-catenin-dependent transactivation in SW480 colon carcinoma cells and its nuclear localization can be inhibited by overexpressing N-cadherin or alpha-catenin. The results indicate that (a) plakoglobin and beta-catenin differ in their nuclear translocation and complexing with LEF-1 and vinculin; (b) LEF-1-dependent transactivation is preferentially driven by beta-catenin; and (c) the cytoplasmic partners of beta-catenin, cadherin and alpha-catenin, can sequester it to the cytoplasm and inhibit its transcriptional activity.
Figures
Figure 1
Schematic representation of catenin constructs used in this study. The molecules were tagged either with the hemagglutinin tag (HA) at the NH2 terminus, or with the VSV-G protein tag (VSV) at the COOH terminus. Numbers 1–13 represent armadillo repeats in β-catenin and plakoglobin with a nonrepeat region (ins) between repeats 10 and 11. Mutant plakoglobin and β-catenin lacking the COOH transactivation domain (HA plakoglobin 1-ins; HA β-catenin 1-ins) were also constructed. An HA-tagged α-catenin that lacks the β-catenin–binding domain was also prepared (HA α_-catenin Δβ_). The COOH-terminal (C-term) transactivation domains of β-catenin and plakoglobin were fused to the DNA binding domain of Gal4 (Gal4DBD) to allow assessment of their transactivation potential.
Figure 2
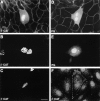
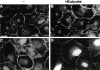
Nuclear localization of β-catenin and plakoglobin transiently transfected into MDCK cells. MDCK cells transfected with VSV-tagged β-catenin (A–C and F, β-CAT) or VSV-tagged plakoglobin (D and E, PG) were immunostained with either monoclonal anti–β-catenin antibody (A), anti-plakoglobin antibody (D), or anti–VSV-tag antibody (B, C, E, and F) and Cy3-labeled secondary antibody, 36 h after transfection. Note the nuclear localization of β-catenin and plakoglobin when overexpressed at high levels in MDCK cells (A–E), and of β-catenin at junctions when expressed at low level (F). Bar, 10 μm.
Figure 3
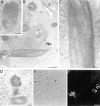
Electronmicroscopy characterization of β-catenin and vinculin-containing nuclear structures in β-catenin–transfected cells. 293-T cells transfected with β-catenin were fixed and processed for (A) conventional TE microscopy, or the β-catenin–induced nuclear structures were identified by cryo-EM with antibodies to β-catenin (B and C) or vinculin (D) using secondary antibodies bound to 10-nm gold particles. The nuclear structures were also visualized by phase (E) and immunofluorescence with anti–β-catenin antibodies (F). Arrows in E point to nuclear structures decorated by anti–β-catenin antibody (F). Nu, nucleus. Bars: (A, C, and D) 0.2 μm; (B) 1 μm; (F) 10 μm.
Figure 7
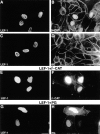
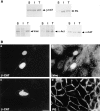
Nuclear translocation of β-catenin but not plakoglobin by LEF-1 overexpression. MDCK cells were transfected with either LEF-1 (A–D), with LEF-1 together with β-catenin (E and F), or with LEF-1 and plakoglobin (G and H). The cells were doubly stained with antibodies against LEF-1 (A, C, E, and G) and antibodies to β-catenin (B) or plakoglobin (D). In doubly-transfected cells (E–H), the transfected β-catenin (F) and plakoglobin (H) were detected by anti-VSV tag antibody. Note that LEF-1 efficiently translocated endogenous β-catenin into the nucleus, but not plakoglobin, whereas in cells transfected with both LEF-1 and plakoglobin or β-catenin, both transfected molecules were localized in the nucleus. Bar, 10 μm.
Figure 4
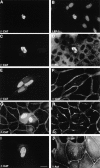
Nuclear translocation of vinculin in β-catenin–transfected cells. MDCK cells were transfected with VSV-tagged β-catenin and doubly stained with antibodies to the VSV tag (A, C, E, and I) or to β-catenin (G), and with antibodies to LEF-1 (B), vinculin (D), α-catenin (F), plakoglobin (H), or α-actinin (J). Note the strong costaining of LEF-1 and vinculin with β-catenin–containing nuclear rods, but not of plakoglobin, α-actinin, or α-catenin. Bar, 10 μm.
Figure 5
Plakoglobin overexpression causes nuclear accumulation of β-catenin. Cells transfected with plakoglobin were doubly stained for plakoglobin (A, C, and E), LEF-1 (B), β-catenin (D), or α-actinin (F). Note that in plakoglobin-transfected cells, β-catenin is translocated into the nucleus. α-Act, α-actinin; β-CAT, β-catenin; PG, plakoglobin. Bar, 10 μm.
Figure 6
Induction of nuclear translocation of β-catenin in stably transfected cells. Control neor HT1080 cells (A and B), and HT1080 cells stably transfected with an NH2-terminal–deleted β-catenin mutant (C and D, ΔN57) were either left untreated (A and C), or treated overnight with sodium butyrate (B and D) to enhance the expression of the transgene. Note the elevation in β-catenin content and its nuclear accumulation in butyrate-treated cells stably expressing ΔN57 β-catenin. Bar, 10 μm.
Figure 8
Differential Triton X-100 solubility of various junctional plaque proteins and nuclear translocation of vinculin in cells overexpressing β-catenin together with LEF-1. (A) Equal volumes of total MDCK cell proteins (T), and Triton X-100–soluble (S) and –insoluble (I) cell fractions, were analyzed by gel electrophoresis and Western blotting with antibodies to β-catenin (β-CAT), plakoglobin (PG), vinculin (vinc), α-actinin (α-Act), and α-catenin (α_-cat_). Note that whereas β-catenin, vinculin, and α-catenin display a large pool of a detergent-soluble fraction, plakoglobin and α-actinin are almost entirely insoluble in Triton X-100. (B) MDCK cells were cotransfected with LEF-1 and β-catenin and doubly stained for β-catenin (a) and vinculin (b), and β-catenin (c) and plakoglobin (d). Note that in cells doubly transfected with β-catenin and LEF-1 vinculin, translocated into the nucleus but plakoglobin remained junctional. Bar, 10 μm.
Figure 9
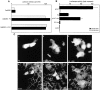
Elevation of β-catenin and plakoglobin content and nuclear localization after treatment with inhibitors of the ubiquitin–proteasome pathway. (A) Balb/C 3T3 cells were treated for 4 h with inhibitors of the ubiquitin–proteasome system: lactacystin (Lact), ALLN, or MG-132, and equal amounts of protein were analyzed by Western blotting with anti–β-catenin antibody. (B) KTCTL60 and KTCTL60-PG cells (stably overexpressing plakoglobin) were treated with 10 and 20 μM MG-132 and probed with anti–β-catenin and plakoglobin antibodies. (C) Northern blot hybridization for β-catenin and plakoglobin in KTCTL60, KTCTL60-PG, and MDCK cells. (D) Balb/C 3T3 and KTCTL60 cells (panels a, c, and e), were treated for 4 h with MG-132 (panels b, d, and f) and stained with antibodies to β-catenin (panels a–d) or plakoglobin (panels e and f). Note the appearance of higher mol wt β-catenin forms in 3T3 cells (bracket in A), the dramatic elevation in β-catenin content of KTCTL60 cells, and the moderate increase in plakoglobin after treatment of KTCTL60-PG with the proteasome inhibitors. Bar, 10 μm.
Figure 10
Activation of Gal4- and LEF-1–driven transcription by β-catenin and plakoglobin. (A) Constructs consisting of the DNA-binding domain of Gal4 (Gal4DBD) fused to the COOH-terminal transactivation domains of β-catenin and plakoglobin were cotransfected with a reporter gene (luciferase) driven by Gal4-responsive sequences into 3T3 cells, and the levels of luciferase activity determined from duplicate transfections (black and white bars). (B) Transactivation of LEF-1 consensus sequence (TOPFLASH)-driven transcription by full-length and truncated β-catenin and plakoglobin in 293 cells. The values (fold increase) were normalized for transfection efficiency by analyzing β-galactosidase activity of cotransfected lacZ, and for LEF-1 specificity with an inactive mutant LEF-1 sequence (FOPFLASH, white bars). (C) Double immunofluorescence for β-catenin (panels b, d, and f) in cells transfected with plakoglobin (a), β-catenin 1-ins (c), and plakoglobin 1-ins (e). Note that chimeras consisting of β-catenin and plakoglobin fused to Gal4DBD were both active in transcription stimulation, but LEF-1–responsive transactivation by β-catenin (and a β-catenin mutant) was much more potent than by full-length plakoglobin, and a COOH-deletion mutant of plakoglobin was inactive in LEF-1–driven transactivation. Full-length plakoglobin and the β-catenin mutant (β-cat 1-ins) were effective in translocating endogenous β-catenin into the nucleus, whereas the plakoglobin mutant (PG 1-ins) was not. Bar, 10 μm.
Figure 11
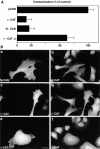
Inhibition of transactivation and nuclear accumulation of β-catenin in SW480 colon carcinoma cells after transfection with N-cadherin or α-catenin. (A) SW480 cells were transfected with either empty vector (pCGN), N-cadherin, α-catenin, or a mutant α-catenin lacking the β-catenin binding site (refer to Fig. 1, HA α_-catenin Δβ_) together with a multimeric LEF-1–binding consensus sequence driving the expression of luciferase. The values of luciferase expression were corrected for transactivation specificity with a mutant LEF-1 consensus sequence, and with β-galactosidase activity for transfection efficiency. (B) Cells were transfected with N-cadherin (panels a and b), α-catenin (panels c and d), or mutant α-catenin (α_-CATΔβ_) (panels e and f) and doubly stained for β-catenin (panels b, d, and f) and N-cadherin (panel a), α-catenin (panel c) and mutant α-catenin (panel e). Note the inhibition of transactivation and cytoplasmic retention of β-catenin in cells transfected with N-cadherin or α-catenin, but not with mutant α-catenin. Bar, 10 μm.
Similar articles
- The dual role of cytoskeletal anchor proteins in cell adhesion and signal transduction.
Ben-Ze'ev A. Ben-Ze'ev A. Ann N Y Acad Sci. 1999;886:37-47. doi: 10.1111/j.1749-6632.1999.tb09398.x. Ann N Y Acad Sci. 1999. PMID: 10667201 Review. - Differential mechanisms of LEF/TCF family-dependent transcriptional activation by beta-catenin and plakoglobin.
Zhurinsky J, Shtutman M, Ben-Ze'ev A. Zhurinsky J, et al. Mol Cell Biol. 2000 Jun;20(12):4238-52. doi: 10.1128/MCB.20.12.4238-4252.2000. Mol Cell Biol. 2000. PMID: 10825188 Free PMC article. - Differential interaction of plakoglobin and beta-catenin with the ubiquitin-proteasome system.
Sadot E, Simcha I, Iwai K, Ciechanover A, Geiger B, Ben-Ze'ev A. Sadot E, et al. Oncogene. 2000 Apr 13;19(16):1992-2001. doi: 10.1038/sj.onc.1203519. Oncogene. 2000. PMID: 10803460 - Regulation of beta-catenin levels and localization by overexpression of plakoglobin and inhibition of the ubiquitin-proteasome system.
Salomon D, Sacco PA, Roy SG, Simcha I, Johnson KR, Wheelock MJ, Ben-Ze'ev A. Salomon D, et al. J Cell Biol. 1997 Dec 1;139(5):1325-35. doi: 10.1083/jcb.139.5.1325. J Cell Biol. 1997. PMID: 9382877 Free PMC article. - Differential molecular interactions of beta-catenin and plakoglobin in adhesion, signaling and cancer.
Ben-Ze'ev A, Geiger B. Ben-Ze'ev A, et al. Curr Opin Cell Biol. 1998 Oct;10(5):629-39. doi: 10.1016/s0955-0674(98)80039-2. Curr Opin Cell Biol. 1998. PMID: 9818174 Review.
Cited by
- Plakoglobin: role in tumorigenesis and metastasis.
Aktary Z, Pasdar M. Aktary Z, et al. Int J Cell Biol. 2012;2012:189521. doi: 10.1155/2012/189521. Epub 2012 Mar 8. Int J Cell Biol. 2012. PMID: 22481945 Free PMC article. - The immunological synapse: the gateway to the HIV reservoir.
Kulpa DA, Brehm JH, Fromentin R, Cooper A, Cooper C, Ahlers J, Chomont N, Sékaly RP. Kulpa DA, et al. Immunol Rev. 2013 Jul;254(1):305-25. doi: 10.1111/imr.12080. Immunol Rev. 2013. PMID: 23772628 Free PMC article. Review. - Complete functional segregation of planarian beta-catenin-1 and -2 in mediating Wnt signaling and cell adhesion.
Chai G, Ma C, Bao K, Zheng L, Wang X, Sun Z, Salò E, Adell T, Wu W. Chai G, et al. J Biol Chem. 2010 Jul 30;285(31):24120-30. doi: 10.1074/jbc.M110.113662. Epub 2010 May 29. J Biol Chem. 2010. PMID: 20511647 Free PMC article. - Membrane-anchored plakoglobins have multiple mechanisms of action in Wnt signaling.
Klymkowsky MW, Williams BO, Barish GD, Varmus HE, Vourgourakis YE. Klymkowsky MW, et al. Mol Biol Cell. 1999 Oct;10(10):3151-69. doi: 10.1091/mbc.10.10.3151. Mol Biol Cell. 1999. PMID: 10512857 Free PMC article. - PAF-AH Catalytic Subunits Modulate the Wnt Pathway in Developing GABAergic Neurons.
Livnat I, Finkelshtein D, Ghosh I, Arai H, Reiner O. Livnat I, et al. Front Cell Neurosci. 2010 May 28;4:19. doi: 10.3389/fncel.2010.00019. eCollection 2010. Front Cell Neurosci. 2010. PMID: 20725507 Free PMC article.
References
- Aberle H, Bierkamp C, Torchard D, Serova O, Wagener T, Natt E, Wirsching J, Heidkämper C, Montagna M, Lynch HT, et al. The human plakoglobin gene localizes on chromosome 17q21 and is subject to loss of heterozygosity in breast and ovarian cancer. Proc Natl Acad Sci USA. 1995;92:6384–6388. - PMC - PubMed
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. - PubMed
- Ben-Ze'ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. - PubMed
- Bierkamp C, Mclaughlin KJ, Schwartz H, Huber O, Kemler R. Embryonic heart and skin defects in mice lacking plakoglobin. Dev Biol. 1996;180:780–785. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous