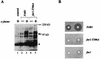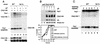Pheromone-dependent G1 cell cycle arrest requires Far1 phosphorylation, but may not involve inhibition of Cdc28-Cln2 kinase, in vivo - PubMed (original) (raw)
Pheromone-dependent G1 cell cycle arrest requires Far1 phosphorylation, but may not involve inhibition of Cdc28-Cln2 kinase, in vivo
A Gartner et al. Mol Cell Biol. 1998 Jul.
Abstract
In yeast, the pheromone alpha-factor acts as an antiproliferative factor that induces G1 arrest and cellular differentiation. Previous data have indicated that Far1, a factor dedicated to pheromone-induced cell cycle arrest, is under positive and negative posttranslational regulation. Phosphorylation by the pheromone-stimulated mitogen-activated protein (MAP) kinase Fus3 has been thought to enhance the binding of Far1 to G1-specific cyclin-dependent kinase (Cdk) complexes, thereby inhibiting their catalytic activity. Cdk-dependent phosphorylation events were invoked to account for the high instability of Far1 outside early G1 phase. To confirm any functional role of Far1 phosphorylation, we undertook a systematic mutational analysis of potential MAP kinase and Cdk recognition motifs. Two putative phosphorylation sites that strongly affect Far1 behavior were identified. A change of serine 87 to alanine prevents the cell cycle-dependent degradation of Far1, causing enhanced sensitivity to pheromone. In contrast, threonine 306 seems to be an important recipient of an activating modification, as substitutions at this position abolish the G1 arrest function of Far1. Only the phosphorylated wild-type Far1 protein, not the T306-to-A substitution product, can be found in stable association with the Cdc28-Cln2 complex. Surprisingly, Far1-associated Cdc28-Cln2 complexes are at best moderately inhibited in immunoprecipitation kinase assays, suggesting unconventional inhibitory mechanisms of Far1.
Figures
FIG. 1
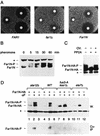
Far1N is phosphorylated in response to pheromone. (A) To measure pheromone sensitivity, halo assays were performed with FAR1 cells (K2149; left panel) and _far1_Δ cells (K2166) transformed with YCplac122 (middle panel) or pGA2046 (FAR1N; right panel). Pheromone concentrations on the filter discs were 1, 0.2, and 0.04 μg, respectively. Photographs were taken after incubation of plates at 30°C for 2 days. (B) To measure Far1N phosphorylation (as revealed by altered mobility), Western blotting was performed with Far1N-containing extracts. Cells were treated with αF for the specified times. Far1N-P indicates the phosphorylated form of Far1N. ∗ marks an unspecific band recognized by the polyclonal anti-Far1 antiserum. Cells used were _far1_Δ (K2166) transformed with FAR1N expressing pGA2042. (C) To measure the phosphatase sensitivity of Far1N-HA (pGA2214) from αF-treated cells, Far1N-HA was immunoprecipitated. The immunoprecipitate was then treated either with PP2A alone (lane 2) or with both PP2A and the phosphatase inhibitor okadaic acid (OV.). ∗ marks an unspecific band. (D) Far1N-HA-T306 phosphorylation depends on upstream components of the pheromone response pathway. Far1N-HA and Cln2-Myc containing extracts were prepared, and coprecipitation experiments were performed as described for Fig. 4A. The upper panel shows the detection of Far1N-HA in a Western blot (W.) of the total cell extract. In the lower panel, coprecipitated (Co-IP) Far1N-HA is shown. ∗ indicates an unspecific band. Strains used were _ste12_Δ (GA161; lanes 1 to 3), WT (GA314; lanes 4 to 6), fus3-A (a loss-of-function mutation of FUS3 that behaves like _fus3_Δ) _kss1_Δ (GA341; lanes 7 to 9), and _ste7_Δ (GA224; lanes 10 to 12).
FIG. 2
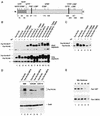
Mutational determination of potential Far1N phosphorylation sites. (A) Diagram showing all SP and TP sites within Far1N. Sites whose mutation leads to a biological phenotype are indicated by bold lines. (B) Western analysis of Far1N-HA substitution mutants. Cells exposed to αF (30 min) are indicated by +. Far1N-HA indicates the phosphorylated version of Far1N-HA. * marks an unspecific band which is recognized by HA antibodies and which was used as internal control to ensure equal loading. The upper and lower panels show short (1-min) and long (5-min) exposures of the Western blot. The following cells were analyzed. GA314 (Mata far1Δ cln2Δ) was transformed with a Cln2-Myc expression plasmid (pGA2236). Furthermore, this strain contained plasmids expressing FAR1N-HA (pGA2214; lanes 1 and 2), FAR1N-HA-T306A (pGA2215; lanes 3 and 4), FAR1N-HA-Q (pGA2216; lanes 5 and 6), FAR1N-HA-Q-T306A (pGA2217; lanes 7 and 8), FAR1N-HA-S87A (pGA2218; lanes 9 and 10), and FAR1N-HA-S87A-T306A (pGA2219) (lanes 11 and 12). “Q” in Far1N-HAQ and Far1N-HA-Q-T306A stands for quadruple substitution, which includes the substitutions S87A, S114A, S341V, and S346A. (C) Far1N-HA-T306S is phosphorylated in response to pheromone. Mata _far1_Δ cells (K2166) transformed with FAR1N-HA (pGA2214; lanes 1, 2, and 7), FAR1N-HA-T306A (pGA2215; lanes 3 and 4), and FAR1N-HA-T306S (lanes 5 and 6) were analyzed by Western blotting. αF induction is indicated by +. (D) S87 phosphorylation is CDC28 dependent and results in cell cycle-specific Far1 degradation. Extracts were prepared from cycling cells (lanes 1 and 2) and from G1 and M-arrested cells (lanes 4 and 5 and lanes 6 and 7, respectively). Strains used for the experiment were K699 (WT), GA250 (cdc15), and K3446 (cdc28-13). Cell cycle arrest was achieved via arresting cells by incubation at the restrictive temperature for 3 h, and uniform cell cycle arrest was confirmed by microscopic examination of cells. A Western blot to detect Far1-HA is shown in the upper panel. Equal loading was confirmed by Ponceau staining of the blot (data not shown). To assess the quality of the extracts, a parallel Western blot was prepared and probed with Swi6 antibodies. (E) Far1 S87A has a decreased turnover. Full-length FAR1 and FAR1-S87A were expressed in a cycling cell population from the GAL promoter by continuous galactose induction. Far1 (detected via anti-Far1 antibodies) is shown after inactivation of the GAL promoter as indicated. Plasmids used were JM306 (GAL-FAR1) and FC361 (GAL-FAR1-S87A).
FIG. 3
Pheromone sensitivity of Far1N-HA substitution mutants. (A) Plate assay. Various _FAR1N-HA_-expressing plasmids were transformed into a _far1_Δ strain (K21669), and pheromone sensitivity was measured by spotting a serial dilution of cells onto plates containing the indicated concentrations of αF. Plasmids used were pGA2214 (FAR1N-HA), pGA2215 (FAR1N-HA-T306A), pGA2218 (FAR1N-HA-S87A), pGA2219 (FAR1N-HA-S87A,T306A), pGA2216 2216 (FAR1N-HA-Q), and pGA2217 (FAR1N-HA-Q-T306A). Q in FAR1N-HA-Q indicates quadruple substitution, which encompasses S87A, S114A, S341V, and S346A. Photographs were taken after 2 days of incubation at 30°C. (B) Budding index of _far1_Δ cells transformed with various _FAR1N-HA_-expressing plasmids (see above) after 0, 1, 2, and 3 h of αF treatment.
FIG. 4
The cell cycle arrest function of Far1 is abolished in full-length Far1-T306A as in Far1N-HA-T306A. (A) Far1 and Far1-T306A phosphorylation was detected by Western blotting of total-cell extract. Cells used were _far1_Δ (GA305) transformed with YCPlac22 (lane 1) or FAR1 (pGA2274; lanes 2 and 3) and far1-T306A (pGA2275; lanes 4 and 5) plasmids. Cells were treated with αF (1 μg/ml) for 15 min (lanes 1, 3, and 5). The arrow indicates Far1 protein. Note that the pheromone-dependent shift (as indicated by the broad signal) of Far1 is much more prominent than in Far1-T306A. * marks an unspecific band recognized by the polyclonal Far1 antiserum. The band seen below the 97-kDa marker seems to be a degradation product of Far1. (B) Pheromone sensitivity was measured by halo assay with a _far1_Δ strain (GA305) transformed with YCPlac22 (lower panel), far1-T306A (pGA2275; middle panel), and FAR1 (pGA2274; upper panel) plasmids. Pheromone concentrations on filter discs were 0.3 and 1 μg. Photos were taken after incubation of the plates at 30°C for 2 days.
FIG. 5
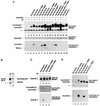
Pheromone-induced Far1N-HA-T306 phosphorylation is required for the pheromone-dependent interaction of Far1N-HA with Cln2-Myc. (A) Coprecipitation of Far1N-HA derivatives with Cln2-Myc. Total extracts from the indicated cells (described in Fig. 2) were generated. Far1N-HA derivatives (from the total-cell extracts) were analyzed by Western blotting (upper panel). After immunoprecipitation of Cln2-Myc by monoclonal anti-Myc antibodies, Cln2-Myc (middle panel) and coprecipitated Far1-HA derivatives (lower panel) were detected by Western blotting. αF induction and the presence of Cln2-Myc are indicated by +. AB (hc.), antibody (heavy chain). (B) Only phosphorylated Far1-HA coprecipitates with Cln2-Myc. Western blotting and immunoprecipitation were performed as for panel A, and Western extract and an immunoprecipitate were loaded on a single gel next to each other as indicated. (C) Coprecipitation of Cln2-HA with Far1N-Myc. Cln2-HA from the total extract was analyzed by Western blotting (top panel) after immunoprecipitation of Far1N-Myc. Both Far1N-Myc (middle panel) and coprecipitated Cln2-HA were detected by Western blotting. For the experiment, we used GA314 (far1Δ cln2Δ) cells which were transformed with pGA2238 (expressing Cln2-HA) and with pGA2209 and pGA2210 (expressing Far1N-Myc [lanes 1 and 2] and Far1N-Myc-T306A [lanes 3 and 4], respectively). (D) Coprecipitation of Far1N-HA derivatives in a FAR1 (GA367) background. The precipitation was performed as for panel A except that all cells were treated with αF for 1 h. Uniform, αF-dependent cell cycle arrest was monitored after further incubating cells for 1.5 h with αF, and cells were fixed for documentation. The upper panel shows the detection of Far1N-HA derivatives from total-cell extracts. In the lower panel, coprecipitated Far1N-HA derivatives were detected after immunoprecipitation of Cln2-Myc.
FIG. 6
Specific Cln2-HA kinase activity is not significantly affected by pheromone-dependent Far1 association. (A) Cln2-HA kinase activity was measured after immunoprecipitation with anti-HA antibodies (upper panel). Far1 indicates coprecipitated Far1 which is phosphorylated by Cln2-HA. The substrate for the kinase reaction was a bacterially produced Far1 peptide encoding aa 51 to 301 (5). The same results were obtained if H1 was used as a substrate or if the extraction buffer described by Peter and Herskowitz (34) was used (data not shown). Equal precipitation of Cln2-HA was ensured by Western blotting of the immunoprecipitate. AB., antibody (heavy chain). The strains used for the experiment were K2149 (WT) and K2166 (_far1_Δ). Both strains were transformed with pTP411, which allows the expression of HA-tagged CLN2 from the CLN3 promoter. (B) GAL1::FAR1 (lanes 1 and 2), GAL1::FAR1 GAL1::CLN2-HA (lanes 3 and 4), and GAL1::FAR1Δ30 GAL1::CLN2-HA (lanes 5 and 6) strains were grown in SCGal-Trp medium and incubated in mating factor or left untreated for 2.5 h. Cln2-HA was immunoprecipitated, and immunoprecipitates assayed for histone H1 kinase activity. In parallel, precipitated Cln2-HA protein and associated Far1 protein were determined by Western blotting. The percentage of unbudded cells in mating factor-treated cultures is indicated in the lower panel. Note that effective cell cycle arrest continued for at least 1 h after the time at which Cln2-associated kinase and Far1 binding were determined. (C) The association between Far1 and Cln2-HA is unaffected by repeated washing of the immunoprecipitate. Kinase assays were performed as described for panel A except that washing of the immunoprecipitate was repeated as indicated.
FIG. 7
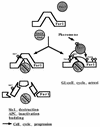
Far1 is regulated by positive and negative phosphorylation events. Pheromone treatment results in the activation of the Fus3 kinase, which in turn causes the phosphorylation of Far1 at T306 and possibly at T63 (left diagram). This phosphorylation event is necessary for the binding of Far1 to the Cln2-Cdc28 kinase. This interaction results into the functional inactivation of the Cln2-Cdc28 cell cycle-promoting activity and results into a G1 cell cycle arrest (right diagram). The detailed molecular consequences of the Far1 Cln2-Cdc28 interaction will be the subject of further studies. Cdc28-dependent S87 phosphorylation counteracts Far1 activation by targeting the protein for destruction (left diagram). As a consequence, negative regulation on the Cln2-Cdc28 complex is alleviated and critical phosphorylation events needed for cell cycle progression can be executed.
Similar articles
- Direct inhibition of the yeast cyclin-dependent kinase Cdc28-Cln by Far1.
Peter M, Herskowitz I. Peter M, et al. Science. 1994 Aug 26;265(5176):1228-31. doi: 10.1126/science.8066461. Science. 1994. PMID: 8066461 - FAR1 links the signal transduction pathway to the cell cycle machinery in yeast.
Peter M, Gartner A, Horecka J, Ammerer G, Herskowitz I. Peter M, et al. Cell. 1993 May 21;73(4):747-60. doi: 10.1016/0092-8674(93)90254-n. Cell. 1993. PMID: 8500168 - Far1 and Fus3 link the mating pheromone signal transduction pathway to three G1-phase Cdc28 kinase complexes.
Tyers M, Futcher B. Tyers M, et al. Mol Cell Biol. 1993 Sep;13(9):5659-69. doi: 10.1128/mcb.13.9.5659-5669.1993. Mol Cell Biol. 1993. PMID: 8395009 Free PMC article. - Regulation of cyclin-substrate docking by a G1 arrest signaling pathway and the Cdk inhibitor Far1.
Pope PA, Bhaduri S, Pryciak PM. Pope PA, et al. Curr Biol. 2014 Jun 16;24(12):1390-1396. doi: 10.1016/j.cub.2014.05.002. Epub 2014 Jun 5. Curr Biol. 2014. PMID: 24909323 Free PMC article. - MAPK cell-cycle regulation in Saccharomyces cerevisiae and Candida albicans.
Correia I, Alonso-Monge R, Pla J. Correia I, et al. Future Microbiol. 2010 Jul;5(7):1125-41. doi: 10.2217/fmb.10.72. Future Microbiol. 2010. PMID: 20632810 Review.
Cited by
- A comprehensive molecular interaction map of the budding yeast cell cycle.
Kaizu K, Ghosh S, Matsuoka Y, Moriya H, Shimizu-Yoshida Y, Kitano H. Kaizu K, et al. Mol Syst Biol. 2010 Sep 21;6:415. doi: 10.1038/msb.2010.73. Mol Syst Biol. 2010. PMID: 20865008 Free PMC article. Review. - Site-specific regulation of the GEF Cdc24p by the scaffold protein Far1p during yeast mating.
Wiget P, Shimada Y, Butty AC, Bi E, Peter M. Wiget P, et al. EMBO J. 2004 Mar 10;23(5):1063-74. doi: 10.1038/sj.emboj.7600123. Epub 2004 Feb 26. EMBO J. 2004. PMID: 14988725 Free PMC article. - Prediction of cyclin-dependent kinase phosphorylation substrates.
Chang EJ, Begum R, Chait BT, Gaasterland T. Chang EJ, et al. PLoS One. 2007 Aug 1;2(7):e656. doi: 10.1371/journal.pone.0000656. PLoS One. 2007. PMID: 17668044 Free PMC article. - Cdc48p is required for the cell cycle commitment point at Start via degradation of the G1-CDK inhibitor Far1p.
Fu X, Ng C, Feng D, Liang C. Fu X, et al. J Cell Biol. 2003 Oct 13;163(1):21-6. doi: 10.1083/jcb.200307025. J Cell Biol. 2003. PMID: 14557244 Free PMC article. - Fus3p and Kss1p control G1 arrest in Saccharomyces cerevisiae through a balance of distinct arrest and proliferative functions that operate in parallel with Far1p.
Cherkasova V, Lyons DM, Elion EA. Cherkasova V, et al. Genetics. 1999 Mar;151(3):989-1004. doi: 10.1093/genetics/151.3.989. Genetics. 1999. PMID: 10049917 Free PMC article.
References
- Barral Y, Jentsch S, Mann C. G1 cyclin turnover and nutrient uptake are controlled by a common pathway in yeast. Genes Dev. 1995;9:399–409. - PubMed
- Blondel M, Mann C. G2 cyclins are required for the degradation of G1 cyclins in yeast. Nature. 1996;384:279–281. - PubMed
- Chang F, Herskowitz I. Identification of a gene necessary for cell cycle arrest by a negative growth factor of yeast: FAR1 is an inhibitor of G1 cyclin, CLN2. Cell. 1990;63:999–1011. - PubMed
- Clark-Lewis I, Sanghera J S, Pelech S L. Definition of a consensus sequence for peptide substrate recognition by p44mpk, the meiosis activated myelin basic protein kinase. J Biol Chem. 1991;266:15180–15184. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases