Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein - PubMed (original) (raw)
Regulation of transcription by hypoxia requires a multiprotein complex that includes hypoxia-inducible factor 1, an adjacent transcription factor, and p300/CREB binding protein
B L Ebert et al. Mol Cell Biol. 1998 Jul.
Abstract
Molecular adaptation to hypoxia depends on the binding of hypoxia-inducible factor 1 (HIF-1) to cognate response elements in oxygen-regulated genes. In addition, adjacent sequences are required for hypoxia-inducible transcription. To investigate the mechanism of interaction between these cis-acting sequences, the multiprotein complex binding to the lactate dehydrogenase A (LDH-A) promoter was characterized. The involvement of HIF-1, CREB-1/ATF-1, and p300/CREB binding protein (CBP) was demonstrated by techniques documenting in vitro binding, in combination with transient transfections that test the in vivo functional importance of each protein. In both the LDH-A promoter and the erythropoietin 3' enhancer, formation of multiprotein complexes was analyzed by using biotinylated probes encompassing functionally critical cis-acting sequences. Strong binding of p300/CBP required interactions with multiple DNA binding proteins. Thus, the necessity of transcription factor binding sites adjacent to a HIF-1 site for hypoxically inducible transcription may be due to the requirement of p300 to interact with multiple transcription factors for high-affinity binding and activation of transcription. Since it has been found to interact with a wide range of transcription factors, p300 is likely to play a similar role in other genes, mediating interactions between DNA binding proteins, thereby activating stimulus-specific and tissue-specific gene transcription.
Figures
FIG. 1
Summary of probes. EMSA probes, labeled L24-HIF and L24-CRE, biotinylated probes (= = =), and biotin label (∗) are indicated. (A) The CRE (site A), HIF-1 consensus sequence (sites B and C), and TATA consensus sequence are underlined. The sequence of mutations in sites A, B, and C are shown below their respective sites. (B) The HIF-1 site (site A) and both half sites of the direct repeat steroid hormone receptor consensus sequence (site B) are underlined. Mutations in sites A and B are shown below the sites; site B is mutated by two base pairs in two locations.
FIG. 2
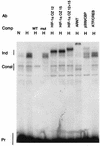
Binding of proteins to the HIF-1 site. EMSAs were performed with the L24-HIF probe with the addition of nuclear extract from HeLa cells incubated in normoxia (N) or hypoxia (H). Inducible (Ind) and constitutive (Const) complexes, as well as the free probe (Pr), are labeled. The addition of competitor (Comp) or antibody (Ab) is noted. OZ12 and OZ15 are monoclonal antibodies to HIF-1α; the combination of both antibodies caused an additive supershift. Supershifts were also observed with an ARNT polyclonal and a p300/CBP monoclonal antibody (AC240) (4) but not with an ATF-1/CREB-1 monoclonal antibody (25C10G; Santa Cruz). WT, wild type; mut, mutant.
FIG. 3
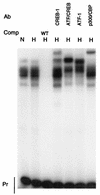
Binding of proteins to the CRE. EMSAs were performed with the L24-CRE probe with the addition of HeLa nuclear extract from normoxic (N) or hypoxic (H) cells. Free probe (Pr), competitors (Comp), and antibodies (Ab) are noted. Supershifts were observed with antibodies to CREB-1 (C-21; Santa Cruz), ATF-1/CREB-1 (25C10G; Santa Cruz), ATF-1 (C41-5.1; Santa Cruz), and p300/CBP. WT, wild type.
FIG. 4
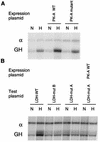
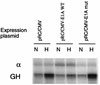
PK-A expression increases hypoxic inducibility of the LDH-A promoter. Cells were cotransfected with a test plasmid, encoding the LDH-A promoter and GH reporter gene, and a control plasmid, encoding the α-globin promoter (α) and gene. Transfected cells were incubated in parallel in normoxia (N) and hypoxia (H), and expression of GH and α-globin was analyzed by RNase protection assay. (A) Expression of wild-type (WT) PK-A increased hypoxic induction of the GH reporter, while expression of a functionally inactive PK-A mutant did not affect hypoxic induction. (B) Mutations in either site B (HIF-1) or site A (CRE) abrogated hypoxic induction, and mutation of site A blocked the effect of PK-A as well.
FIG. 5
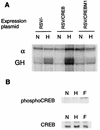
Effect of CREB overexpression and CREBM1 on the LDH-A promoter. (A) Hypoxic induction of the transfected GH reporter, relative to α-globin control (α), was measured by RNase protection assay. Hypoxic induction was increased by cotransfection of a CREB expression plasmid and decreased by expression of a dominant negative protein, CREBM1. (B) To test whether hypoxia influences the phosphorylation of CREB, Western blot analyses were performed with nuclear extracts from HeLa cells incubated in normoxia (N), hypoxia (H), or 20 μM forskolin (F). Blots were probed with an anti-phospho-CREB antibody (UBI) or an anti-CREB antibody (25C10G; Santa Cruz).
FIG. 6
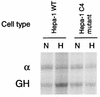
The LDH-A promoter is not regulated by oxygen in ARNT-deficient cells. Hypoxic induction of the transfected GH reporter, relative to α-globin control, was measured by RNase protection assay. Hypoxic inducibility of the LDH-A promoter was intact in wild-type Hepa-1 cells, but in the ARNT-deficient cell line Hepa-1 c4, the LDH-A promoter was not responsive to hypoxia. For abbreviations, see the legend to Fig. 4.
FIG. 7
Requirement of p300 for oxygen regulation. Cells were cotransfected with a vector expressing wild-type (WT) E1A or a vector expressing a mutant (mut) form of E1A which does not bind to p300. Hypoxic induction of the reporter, relative to α-globin control (α), was measured by RNase protection assay. Expression of E1A nearly abolished induction by hypoxia, whereas the mutated E1A did not affect hypoxic inducibility of the LDH-A promoter.
FIG. 8
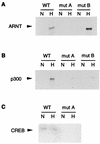
Hypoxically inducible complex in the LDH-A promoter. Nuclear extract from normoxic (N) or hypoxic (H) HeLa cells was incubated with a double-stranded, biotinylated probe bound to streptavidin-coated magnetic beads. Multiprotein complexes were purified by using a magnet, washed, and analyzed by Western blotting. Binding was compared between wild-type (WT) LDH-A probe and probes in which functionally critical sites were mutated. In the probe denoted mut BC, both sites B and C were mutated; in mut ABC, sites A, B, and C were mutated. Western blots were probed with antibodies to CREB-1/ATF-1 (25C10G; Santa Cruz) (A), ARNT (B), or p300 (C and D). In panel C, protein complexes bound to the probe and beads were washed with low stringency; in panel D, protein complexes were washed with high stringency.
FIG. 9
Hypoxically inducible complex in the Epo 3′ enhancer. Nuclear extract from normoxic (N) or hypoxic (H) HeLa cells was incubated with a biotinylated probe derived from the Epo 3′ enhancer. Multiprotein complexes were pulled down and analyzed on Western blots probed with antibodies to ARNT (A), p300 (B), or CREB-1/ATF-1 (25C10G; Santa Cruz) (C). Binding to the wild-type (WT) probe was compared with binding to probes with mutations in the HIF-1 site (mut A) or HRE (mut B). p300 binding was tested under high-stringency wash conditions.
Similar articles
- Molecular regulation of the endothelin-1 gene by hypoxia. Contributions of hypoxia-inducible factor-1, activator protein-1, GATA-2, AND p300/CBP.
Yamashita K, Discher DJ, Hu J, Bishopric NH, Webster KA. Yamashita K, et al. J Biol Chem. 2001 Apr 20;276(16):12645-53. doi: 10.1074/jbc.M011344200. Epub 2001 Jan 22. J Biol Chem. 2001. PMID: 11278891 - Hypoxic regulation of lactate dehydrogenase A. Interaction between hypoxia-inducible factor 1 and cAMP response elements.
Firth JD, Ebert BL, Ratcliffe PJ. Firth JD, et al. J Biol Chem. 1995 Sep 8;270(36):21021-7. doi: 10.1074/jbc.270.36.21021. J Biol Chem. 1995. PMID: 7673128 - Erythropoietin gene regulation depends on heme-dependent oxygen sensing and assembly of interacting transcription factors.
Huang LE, Ho V, Arany Z, Krainc D, Galson D, Tendler D, Livingston DM, Bunn HF. Huang LE, et al. Kidney Int. 1997 Feb;51(2):548-52. doi: 10.1038/ki.1997.76. Kidney Int. 1997. PMID: 9027736 Review. - Structural and functional analysis of hypoxia-inducible factor 1.
Semenza GL, Agani F, Booth G, Forsythe J, Iyer N, Jiang BH, Leung S, Roe R, Wiener C, Yu A. Semenza GL, et al. Kidney Int. 1997 Feb;51(2):553-5. doi: 10.1038/ki.1997.77. Kidney Int. 1997. PMID: 9027737 Review.
Cited by
- Functional and transcriptional induction of aquaporin-1 gene by hypoxia; analysis of promoter and role of Hif-1α.
Abreu-Rodríguez I, Sánchez Silva R, Martins AP, Soveral G, Toledo-Aral JJ, López-Barneo J, Echevarría M. Abreu-Rodríguez I, et al. PLoS One. 2011;6(12):e28385. doi: 10.1371/journal.pone.0028385. Epub 2011 Dec 7. PLoS One. 2011. PMID: 22174795 Free PMC article. - Loss of pVHL is sufficient to cause HIF dysregulation in primary cells but does not promote tumor growth.
Mack FA, Rathmell WK, Arsham AM, Gnarra J, Keith B, Simon MC. Mack FA, et al. Cancer Cell. 2003 Jan;3(1):75-88. doi: 10.1016/s1535-6108(02)00240-4. Cancer Cell. 2003. PMID: 12559177 Free PMC article. - Cancer and altered metabolism: potential importance of hypoxia-inducible factor and 2-oxoglutarate-dependent dioxygenases.
Kaelin WG Jr. Kaelin WG Jr. Cold Spring Harb Symp Quant Biol. 2011;76:335-45. doi: 10.1101/sqb.2011.76.010975. Epub 2011 Nov 16. Cold Spring Harb Symp Quant Biol. 2011. PMID: 22089927 Free PMC article. Review. - Hypoxia-inducible factor (HIF) network: insights from mathematical models.
Cavadas MA, Nguyen LK, Cheong A. Cavadas MA, et al. Cell Commun Signal. 2013 Jun 10;11(1):42. doi: 10.1186/1478-811X-11-42. Cell Commun Signal. 2013. PMID: 23758895 Free PMC article. - Transcriptional regulation by hypoxia inducible factors.
Dengler VL, Galbraith M, Espinosa JM. Dengler VL, et al. Crit Rev Biochem Mol Biol. 2014 Jan-Feb;49(1):1-15. doi: 10.3109/10409238.2013.838205. Epub 2013 Oct 7. Crit Rev Biochem Mol Biol. 2014. PMID: 24099156 Free PMC article. Review.
References
- Avantaggiati M L, Ogryzko V, Gardner K, Giordano A, Levine A S, Kelly K. Recruitment of p300/CBP in p53-dependent signal pathways. Cell. 1997;89:1175–1184. - PubMed
- Bhattacharya S, Eckner R, Grossman S, Oldread E, Arany Z, D’Andrea A, Livingston D M. Cooperation of Stat2 and p300/CBP in signalling induced by interferon-alpha. Nature. 1996;383:344–347. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous