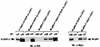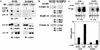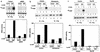Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase - PubMed (original) (raw)
Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase
M Takahashi-Tezuka et al. Mol Cell Biol. 1998 Jul.
Abstract
Gab1 has structural similarities with Drosophila DOS (daughter of sevenless), which is a substrate of the protein tyrosine phosphatase Corkscrew. Both Gab1 and DOS have a pleckstrin homology domain and tyrosine residues, potential binding sites for various SH2 domain-containing adapter molecules when they are phosphorylated. We found that Gab1 was tyrosine phosphorylated in response to various cytokines, such as interleukin-6 (IL-6), IL-3, alpha interferon (IFN-alpha), and IFN-gamma. Upon the stimulation of IL-6 or IL-3, Gab1 was found to form a complex with phosphatidylinositol (PI)-3 kinase and SHP-2, a homolog of Corkscrew. Mutational analysis of gp130, the common subunit of IL-6 family cytokine receptors, revealed that neither tyrosine residues of gp130 nor its carboxy terminus was required for tyrosine phosphorylation of Gab1. Expression of Gab1 enhanced gp130-dependent mitogen-activated protein (MAP) kinase ERK2 activation. A mutation of tyrosine 759, the SHP-2 binding site of gp130, abrogated the interactions of Gab1 with SHP-2 and PI-3 kinase as well as ERK2 activation. Furthermore, ERK2 activation was inhibited by a dominant negative p85 PI-3 kinase, wortmannin, or a dominant negative Ras. These observations suggest that Gab1 acts as an adapter molecule in transmitting signals to ERK MAP kinase for the cytokine receptor gp130 and that SHP-2, PI-3 kinase, and Ras are involved in Gab1-mediated ERK activation.
Figures
FIG. 1
Tyrosine phosphorylation of Gab1 and its association with SHP-2 and the p85 subunit of PI-3 kinase. (a) IL-6 and IL-3 induce tyrosine phosphorylation of Gab1. HepG2 and TF-1 cells (106) were stimulated with IL-6 (100 ng/ml) and IL-3 (10 ng/ml), respectively, for the indicated period. Gab1 was immunoprecipitated (IP) with an anti-Gab1 antibody (α-Gab1), transferred to a membrane, and immunoblotted (IB) with antiphosphotyrosine (α-PY), anti-Gab1, anti-SHP-2, or anti-p85 antibodies. The arrows indicate the locations of Gab1, SHP-2, and p85. Abbreviations apply to all figures. (b) IFN-α and -γ induce tyrosine phosphorylation of Gab1. Hep3B cells were stimulated with IFN-α or IFN-γ (100 ng/ml) for 15 min or not stimulated (−). The Gab1 immunoprecipitates were analyzed by immunoblotting with antiphosphotyrosine (upper panel) or anti-Gab1 (lower panel) antibodies. Locations of Gab1 are indicated by arrows. (c) Gab1 associates with SHP-2 and PI-3 kinase in response to IL-6 or IL-3. HepG2 and TF-1 cells were stimulated with IL-6 and IL-3, respectively. gp130, SHP-2, Gab1, the p85 subunit of PI-3 kinase, or Grb2 was immunoprecipitated with anti-gp130, anti-SHP-2, anti-Gab1, anti-p85, or anti-Grb2 antibodies, respectively, and blotted with antiphosphotyrosine, p85, SHP-2, or Gab1 antibodies as indicated. The 160-kDa bands (marked by a dot) in the p85 immunoprecipitates on antiphosphotyrosine blotting were nonspecific, since they were not recognized by anti-gp130 antibody (data not shown), and the 80-kDa bands in the Grb2-immunoprecipitates (marked by an asterisk) were also nonspecific since they migrated differently from p85. Note that a relatively large amount of Gab1 was detected in the p85 immunoprecipitates from the unstimulated HepG2 cells. This is likely due to a low level of tyrosine phosphorylation of Gab1 in the unstimulated cells and the fact that phosphorylated Gab1 was concentrated by the associated p85. (d) PI-3 kinase activity associates with Gab1 and SHP-2. HepG2 cells (106) were stimulated with IL-6 (100 ng/ml) for 10 min (+) or not stimulated (−). Lysates were immunoprecipitated with anti-gp130, anti-Gab1, anti-SHP-2, anti-p85, or antiphosphotyrosine antibodies (near-saturation amount), and their associated PI-3 kinase activities were determined by in vitro kinase reaction using PI as a substrate. PI phosphate (PIP) was separated by thin-layer chromatography as described in Materials and Methods and analyzed by autoradiography. The incorporated radioactivity was quantitated by an image analyzer. Two independent data sets obtained from separate experiments are represented by an autoradiograph (left panel) and a bar graph (right panel). The amounts of p85 in anti-p85, antiphosphotyrosine, or anti-Gab1 antibody immunoprecipitates were analyzed by immunoblotting with the anti-p85 antibody (right panel, bottom). Arrows on the left indicate locations of origin (Ori) and PIP. Numbers on the graph are arbitrary units of PI kinase activity obtained from the image analyzer.
FIG. 2
Interaction between Gab1 and PI-3 kinase, Gab1 and SHP-2, or SHP-2 and PI-3 kinase. PI-3 kinase associates with Gab1 directly and with SHP-2 through Gab1. 293T cells were transfected with the expression vectors for F-Gab1, H-SHP-2, and M-p85 with a JAK1 expression vector as indicated. Cell lysates were immunoprecipitated with anti-Flag (F), -HA (H), or -Myc (M) antibodies, and SHP-2 and p85 protein were blotted with anti-HA (left) and anti-Myc (right) antibodies.
FIG. 3
Tyrosine phosphorylation or the carboxy-terminal region of gp130 is not necessary for tyrosine phosphorylation of Gab1. (a) The carboxy-terminal region of gp130 is not necessary for tyrosine phosphorylation of Gab1. 293T cells stably expressing the chimeric receptor G277 (containing the entire cytoplasmic domain of gp130) or G68 (containing 68 amino acid residues from the membrane) were stimulated with G-CSF (100 ng/ml) for 10 min (+) or left unstimulated (−). Cell lysates were immunoprecipitated with anti-Gab1 or anti-G-CSFR antibodies and immunoblotted with antiphosphotyrosine, anti-Gab1, or anti-G-CSFR antibodies, as indicated. The arrows indicate the locations of Gab1 and the chimeric receptors. (b) Tyrosine phosphorylation of gp130 is not necessary for Gab1 tyrosine phosphorylation. 293T cells were transiently transfected with the expression vectors for G-CSFR–gp130 chimeric receptors, G277 and G-Fall, in which all six tyrosines in the cytoplasmic domain of gp130 were mutated to phenylalanines. Cells were stimulated with G-CSF (+) or left unstimulated (−). Gab1 or the chimeric receptors were immunoprecipitated and blotted with antiphosphotyrosine, anti-Gab1, or anti-G-CSFR antibodies. Arrows indicate the locations of Gab1 and the chimeric receptors.
FIG. 4
Expression of Gab1 enhances gp130-mediated ERK2 MAP kinase activation. (a) Expression of Gab1 and the stimulation of gp130 synergistically induce kinase activity of ERK2. For the analysis of ERK2 activation, 293T cells expressing the G277 chimeric receptor (293T-G277) were transiently transfected with expression vectors for F-ERK2 alone (lane 1), F-ERK2 and a mock control (pcDNA3; lanes 2 and 3), or F-ERK2 and H-Gab1 (lanes 4 and 5). Cells were stimulated with 100 μM tetradecanoyl phorbol acetate (lane 1) or G-CSF (100 ng/ml) for 30 min (+; lanes 3 and 5) or left unstimulated (−; lanes 4 and 6). ERK2 was immunoprecipitated with anti-Flag antibody, and its activity was determined by in vitro kinase assay using MBP as a substrate. Phosphorylated MBP was separated on an SDS-polyacrylamide gel and analyzed by autoradiography. For the analysis of JNK1 activation, 293T-G277 cells were transfected with expression vectors for F-JNK1 and MEKK1 (a positive control; lane 6), F-JNK1 and a mock control (pcDNA3; lanes 7 and 8), or F-JNK1 and H-Gab1 (lanes 9 and 10). Cells were stimulated with G-CSF (+; lanes 8 and 10) or left unstimulated (−; lanes 7 and 9). JNK1 was immunoprecipitated with anti-Flag antibody, and its activity was determined by in vitro kinase assay using GST–c-Jun (1/79) as a substrate. ERK2 and JNK1 expression was detected by immunoblotting with anti-Flag antibodies. Incorporated 32P in MBP was quantified by an imaging analyzer, and the results are shown in a bar graph (lower panel). ERK2 activities in the graph are percentages of MBP-incorporated 32P for ERK2 obtained from unstimulated cells (lane 2). (b) Gab1 activates IL-6-dependent ERK2 activation. HepG2 cells were transiently transfected with expression vectors for F-ERK2 and a mock control (lanes 1 and 2) or F-ERK2 and H-Gab1 (lanes 3 and 4). Cells were stimulated with IL-6 (100 ng/ml) for 30 min (+; lanes 2 and 4) or left unstimulated (−; lanes 1 and 3). Kinase activities and expression levels of F-ERK2 were determined as described above.
FIG. 5
Tyrosine 759, the SHP-2 binding site of gp130, is essential for the interactions between Gab1 and SHP-2, or Gab1 and PI-3 kinase, and Gab1-mediated ERK activation. (a) Interaction between Gab1 and SHP-2 depends on tyrosine 759. 293T cells stably expressing G133 (293T-G133) or G133F2 (293T-G133F2), in which tyrosine 759 was mutated to phenylalanine, were stimulated with G-CSF or left unstimulated. SHP-2 and Gab1 were immunoprecipitated with the specific antibodies and blotted with antiphosphotyrosine, anti-SHP-2, or anti-Gab1 antibodies. The locations of SHP-2, Gab1, and G133 (the chimeric receptor) are indicated by arrows. (b) Interaction between Gab1 and PI-3 kinase depends on tyrosine 759. 293T-G133 and 293T-133F2 cells were transfected with expression vectors for M-p85 and H-Gab1. Cells were stimulated with G-CSF or left unstimulated. Lysates were immunoprecipitated with anti-Myc (for p85) or anti-HA (Gab1) antibodies and analyzed by immunoblotting. Essentially the same results were obtained for the interaction between endogenous p85 and Gab1 (data not shown). (c) Tyrosine 759 of gp130 is necessary for Gab1-dependent ERK2 activation. 293T-G133 and 293T-G133F2 cells were transfected with expression vectors for F-ERK2 (lanes 1, 2, 5, and 6) or F-ERK2 and H-Gab1 (lanes 3, 4, 7, and 8). Cells were stimulated with G-CSF (+; lanes 2, 4, 6, and 8) or not stimulated (−; lanes 1, 3, 5, and 7). ERK2 kinase activities were determined by in vitro kinase assay and are illustrated by an autoradiograph and a bar graph as described for Fig. 6. The expression of ERK2 was analyzed by immunoblotting with anti-Flag antibody. ERK2 activities in the graph are percentages of MBP-incorporated 32P for ERK2 obtained from unstimulated G133F2-expressing cells (lane 5).
FIG. 6
PI-3 kinase and Ras are involved in the Gab1-mediated ERK activation. (a) p85DN inhibits ERK2 activation. 293T-G277 cells were transfected with expression vectors for F-ERK2 (lanes 1 and 2), F-ERK2 and H-Gab1 (lanes 3 and 4), F-ERK2 and dominant negative p85DN (lanes 5 and 6), or F-ERK2, H-Gab1, and p85DN (lanes 7 and 8). The amounts of expression vectors were normalized by addition of a mock control vector, pcDNA3. Cells were stimulated with G-CSF (+; lanes 2, 4, 6, and 8) or left unstimulated (−; lanes 1, 3, 5, and 7). ERK2 activities and expression were determined as described for Fig. 5. ERK2 activities in the graph are percentages of that in unstimulated cells (lane 1). (b) Wortmannin inhibits the Gab1-mediated ERK2 activation. 293T-G277 cells were transfected with expression vectors for F-ERK2 (lanes 1, 2, 5, and 6) or F-ERK2 and H-Gab1 (lanes 3, 4, 7, and 8). Cells were incubated in 100 nM wortmannin for 1 h before and during stimulation (lanes 5 to 8) and then stimulated with G-CSF (+; lanes 2, 4, 6, and 8) or left unstimulated (lanes 1, 3, 5, and 7). ERK2 activities were determined by in vitro kinase assay. (c) RasN17 inhibits ERK2 activation. 293T-G277 cells were transfected with expression vectors for F-ERK2 (lanes 1 and 2), F-ERK2 and H-Gab1 (lanes 3 and 4), F-ERK2 and RasN17 (lanes 5 and 6), or F-ERK2, H-Gab1, and RasN17 (lanes 7 and 8). Cells were stimulated with G-CSF (+; lanes 2, 4, 6, and 8) or left unstimulated (−; lanes 1, 3, 5, and 7). ERK2 activities and expression were determined.
FIG. 7
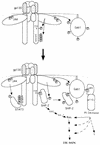
Model of roles of Gab1 in gp130 signaling. For details, see Discussion. MAPK, MAP kinase.
Similar articles
- Gab-family adapter proteins act downstream of cytokine and growth factor receptors and T- and B-cell antigen receptors.
Nishida K, Yoshida Y, Itoh M, Fukada T, Ohtani T, Shirogane T, Atsumi T, Takahashi-Tezuka M, Ishihara K, Hibi M, Hirano T. Nishida K, et al. Blood. 1999 Mar 15;93(6):1809-16. Blood. 1999. PMID: 10068651 - Activation of gp130 transduces hypertrophic signal through interaction of scaffolding/docking protein Gab1 with tyrosine phosphatase SHP2 in cardiomyocytes.
Nakaoka Y, Nishida K, Fujio Y, Izumi M, Terai K, Oshima Y, Sugiyama S, Matsuda S, Koyasu S, Yamauchi-Takihara K, Hirano T, Kawase I, Hirota H. Nakaoka Y, et al. Circ Res. 2003 Aug 8;93(3):221-9. doi: 10.1161/01.RES.0000085562.48906.4A. Epub 2003 Jul 10. Circ Res. 2003. PMID: 12855672 - Gab-family adapter molecules in signal transduction of cytokine and growth factor receptors, and T and B cell antigen receptors.
Hibi M, Hirano T. Hibi M, et al. Leuk Lymphoma. 2000 Apr;37(3-4):299-307. doi: 10.3109/10428190009089430. Leuk Lymphoma. 2000. PMID: 10752981 Review. - The role of Gab family scaffolding adapter proteins in the signal transduction of cytokine and growth factor receptors.
Nishida K, Hirano T. Nishida K, et al. Cancer Sci. 2003 Dec;94(12):1029-33. doi: 10.1111/j.1349-7006.2003.tb01396.x. Cancer Sci. 2003. PMID: 14662016 Free PMC article. Review.
Cited by
- Role of Gab1 in UV-induced c-Jun NH2-terminal kinase activation and cell apoptosis.
Sun Y, Yuan J, Liu H, Shi Z, Baker K, Vuori K, Wu J, Feng GS. Sun Y, et al. Mol Cell Biol. 2004 Feb;24(4):1531-9. doi: 10.1128/MCB.24.4.1531-1539.2004. Mol Cell Biol. 2004. PMID: 14749370 Free PMC article. - Neurons or glia? Can SHP2 know it all?
Coskun V, Zhao J, Sun YE. Coskun V, et al. Sci STKE. 2007 Oct 30;2007(410):pe58. doi: 10.1126/stke.4102007pe58. Sci STKE. 2007. PMID: 17971566 Free PMC article. - The multi-site docking protein Grb2-associated binder 1 (Gab1) enhances interleukin-6-induced MAPK-pathway activation in an SHP2-, Grb2-, and time-dependent manner.
Bongartz H, Gille K, Hessenkemper W, Mandel K, Lewitzky M, Feller SM, Schaper F. Bongartz H, et al. Cell Commun Signal. 2019 Oct 24;17(1):135. doi: 10.1186/s12964-019-0451-2. Cell Commun Signal. 2019. PMID: 31651330 Free PMC article. - A novel positive feedback loop mediated by the docking protein Gab1 and phosphatidylinositol 3-kinase in epidermal growth factor receptor signaling.
Rodrigues GA, Falasca M, Zhang Z, Ong SH, Schlessinger J. Rodrigues GA, et al. Mol Cell Biol. 2000 Feb;20(4):1448-59. doi: 10.1128/MCB.20.4.1448-1459.2000. Mol Cell Biol. 2000. PMID: 10648629 Free PMC article. - Gab1 mediates hepatocyte growth factor-stimulated mitogenicity and morphogenesis in multipotent myeloid cells.
Felici A, Giubellino A, Bottaro DP. Felici A, et al. J Cell Biochem. 2010 Oct 1;111(2):310-21. doi: 10.1002/jcb.22695. J Cell Biochem. 2010. PMID: 20506405 Free PMC article.
References
- Adachi M, Fischer E H, Ihle J, Imai K, Jirik F, Neel B, Pawson T, Shen S-H, Thomas M, Ullrich A, Zhao Z. Mammalian SH2-containing protein tyrosine phosphatases. Cell. 1996;85:15. - PubMed
- Boulton T G, Stahl N, Yancopoulos G D. Ciliary neurotropic factor/leukemia inhibitory factor/interleukin 6/oncostatin M family of cytokines induces tyrosine phosphorylation of a common set of proteins overlapping those induced by other cytokines and growth factors. J Biol Chem. 1994;269:11648–11655. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous