Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia - PubMed (original) (raw)
Induction of caspase-3-like protease may mediate delayed neuronal death in the hippocampus after transient cerebral ischemia
J Chen et al. J Neurosci. 1998.
Abstract
Delayed neuronal death after transient cerebral ischemia may be mediated, in part, by the induction of apoptosis-regulatory gene products. Caspase-3 is a newly characterized mammalian cysteine protease that promotes cell death during brain development, in neuronal cultures, and in other cell types under many different conditions. To determine whether caspase-3 serves to regulate neuronal death after cerebral ischemia, we have (1) cloned a cDNA encoding the rat brain caspase-3; (2) examined caspase-3 mRNA and protein expression in the brain using in situ hybridization, Northern and Western blot analyses, and double-labeled immunohistochemistry; (3) determined caspase-3-like activity in brain cell extracts; and (4) studied the effect of caspase-3 inhibition on cell survival and DNA fragmentation in the hippocampus in a rat model of transient global ischemia. At 8-72 hr after ischemia, caspase-3 mRNA and protein were induced in the hippocampus and caudate-putamen (CPu), accompanied by increased caspase-3-like protease activity. In the hippocampus, caspase-3 mRNA and protein were predominantly increased in degenerating CA1 pyramidal neurons. Proteolytic activation of the caspase-3 precursor was detected in hippocampus and CPu but not in cortex at 4-72 hr after ischemia. Double-label experiments detected DNA fragmentation in the majority of CA1 neurons and selective CPu neurons that overexpressed caspase-3. Furthermore, ventricular infusion of Z-DEVD-FMK, a caspase-3 inhibitor, decreased caspase-3 activity in the hippocampus and significantly reduced cell death and DNA fragmentation in the CA1 sector up to 7 d after ischemia. These data strongly suggest that caspase-3 activity contributes to delayed neuronal death after transient ischemia.
Figures
Fig. 1.
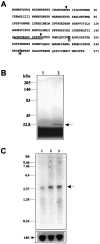
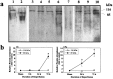
A, Deduced amino acid sequence of rat brain caspase-3 from the cDNA clone. This sequence shares 84.1% identity with the human cpp32β (Fernandes-Alnemri et al., 1994) and 99.3% with the rat brain IRP (Ni et al., 1997). The_boxes_ mark the different amino acids between caspase-3 and the rat brain IRP. Arrowheads indicate the known proen-zyme cleavage sites for caspase-3 (Asp28–Ser29 and Asp175–Ser176) that yield the p17 and p12 active forms. The peptide sequence used to raise the caspase-3 antibody is_underlined_. B, SDS-PAGE analysis of extract of in vitro translation assay product from caspase-3 cDNA. Lane 1, Negative control. cRNA was omitted from the assay. Lane 2, Translation product (arrow) from the caspase-3 cDNA. C, Northern blot analysis of caspase-3 mRNA in the hippocampus after sham operation (lane 1) or 8 hr (lane 2) or 24 hr (lane 3) after ischemia. Total RNA was isolated from the hippocampi (three brains per time point) and electrophoresed through a 1% agarose–formaldehyde gel (20 μg of RNA per_lane_). The only transcription species resulting from hybridizing with the caspase-3 cDNA probe is ∼2.6–2.7 kb (arrow), consistent with the predicted molecular size of rat caspase-3 mRNA. Bottom, The same blot hybridized with the 18S RNA probe as a control for sample loading.
Fig. 2.
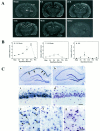
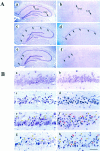
Caspase-3 in situ hybridization.A, Representative autoradiograms through the level of dorsal hippocampus in a sham-operated brain and in brains at 8, 24, 72, and 96 hr after 15 min of global ischemia. Caspase-3 mRNA is slightly increased in the dentate gyrus (DG) granule cell layer 8 hr after ischemia but markedly increased in the hippocampal_CA1_ sector at 24 and 72 hr after ischemia (arrowheads). A section (24 hr after ischemia;Sense) hybridized with the sense cDNA probe results in only background signals. B, Relative caspase-3 mRNA changes in the hippocampal CA1 sector, CA3 sector, and dentate gyrus at 4, 8, 24, 72, and 96 hr after ischemia versus sham controls (n = 3 per group), determined by optical density measurement on autoradiograms. Data are reported as mean ± SEM and represent fold changes in ischemic brains versus sham controls; *p < 0.01, and ** p < 0.001 versus sham controls (ANOVA and post hoc Fisher’s PLSD tests). C, Representative emulsion-coated sections counterstained with cresyl violet from a brain 72 hr after ischemia (a, c, e) and a sham control brain (b, d,f). Caspase-3 mRNA is predominantly increased in the CA1 sector of ischemic hippocampus (a;arrows) compared with the control brain (b). Under a high-power field (400×), increased amounts of silver grains localize to CA1 pyramidal neurons (c) and to scattered neurons in the caudate-putamen (e; arrows) compared with the controls d and f, respectively. The_open squares_ in a and _b_mark the regions from which the high-power views in _c_and d are taken, respectively. g, Sense control in caudate-putamen from the same ischemic brain. Scale bar, 30 μm.
Fig. 3.
Western blot analysis of caspase-3 protein in the hippocampus (Hi), caudate-putamen (CPu), and cortex (Ctx) from brains after sham operation (lane 1) or 4 hr (lane 2), 8 hr (lane 3), 24 hr (lane 4), or 72 hr (lane 5) after ischemia. Immunoreactivity of both caspase-3 precursor protein (32 kDa) and its larger cleavage form (17 kDa) is increased in the hippocampus after ischemia. Immunoreactivity of the 17 kDa cleavage form is also increased in the caudate-putamen but not in the cortex. Graphs, Semiquantitative changes of caspase-3 protein after ischemia, determined by optical density (OD) measurements on Western blot autoradiograms (OD × area). Data are mean ± SEM (n = 4 per time point) and represent fold changes in ischemic brains versus sham controls; *,#p < 0.05 versus sham controls (ANOVA and post hoc Fisher’s PLSD tests).
Fig. 4.
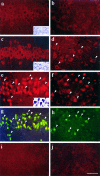
Immunofluorescent images of caspase-3 protein (a–f, i, j) and TUNEL labeling (g, h) in the hippocampal CA1 sector (left) and caudate-putamen (right) after ischemia. Compared with that in the control brain (a, b), caspase-3 immunofuorescence is increased in the cytoplasm of CA1 pyramidal neurons (c) and in scattered neurons in the caudate-putamen (d; arrowheads) at 24 hr after ischemia. Caspase-3 immunofluorescence is increased further in neurons in these regions with both cytosolic and nuclear localization at 72 hr after ischemia (e, f;arrowheads mark representative positive cells). Double-label (TUNEL) in the same sections shows a colocalization of DNA fragmentation (g, h;arrowheads mark representative positive cells) and increased caspase-3 immunofluorescence in most CA1 neurons (e vs g) and in many caudate neurons (f vs h) at 72 hr after ischemia. Note that TUNEL-positive neurons show a condensed and shrunken nucleus. Consecutive sections of c and d incubated with the primary antibody preabsorbed with the synthetic caspase-3 peptide show background fluorescence only (i, j).Insets in a, c, and_e_ show representative cresyl violet staining in the CA1 sector. In keeping with the delayed manner of cell death in this model, CA1 neurons show normal morphology in control brain (a) and in the brain 24 hr after ischemia (c) but show pyknotic changes in the brain 72 hr after ischemia (e). Scale bar, 50 μm.
Fig. 5.
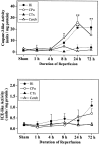
The caspase-3-like (top) and ICE-like protease activity (bottom) in cell extracts from the hippocampus (Hi), caudate-putamen (CPu), cortex (CTx), and cerebellum (Cereb) after sham operation or 1, 4, 8, 24, or 72 hr after ischemia. The protease activities are measured by determining the ability of cell extracts to cleave the colorimetric substrates Ac-DEVD-_p_NA (for caspase-3-like activity) and Ac-YVAD-_p_NA (for ICE-like activity). One unit of protease activity corresponds to the caspase-like activity that cleaves 1 pmol of _p_NA per minute at 37°C at saturating substrate concentrations. Data are presented as mean ± SEM (n = 4–5 per time point); *p< 0.05, and **p < 0.01 versus sham controls (ANOVA and post hoc Fisher’s PLSD tests).
Fig. 6.
Western blot analysis of PARP in the hippocampus and caudate-putamen after ischemia. a, Lanes 1–2, Positive controls for intact PARP (116 kDa) and cleaved PARP (85 kDa) using cell extracts from uninduced human HL60 leukemia cells and HL60 induced to undergo apoptosis, respectively. Lanes 3–6, Cell extracts from the hippocampus after sham operation (lane 3) or 8 hr (lane 4), 24 hr (lane 5), or 72 hr (lane 6) after ischemia. Note that the 85 kDa form of PARP is increased at 72 hr.Lane 7, The same cell extract used in lane 6 incubated with the primary antibody preabsorbed with purified bovine PARP protein (10 μg/ml). Lanes 8–10, Cell extracts from the caudate-putamen after sham operation (lane 8) or 24 hr (lane 9) or 72 hr (lane 10) after ischemia. Note that the 85 kDa form is increased in both ischemic samples. b, Semiquantitative analysis of relative PARP changes in the hippocampus (Hi) and caudate-putamen (CPu) after ischemia. Data are mean ± SEM (n = 3 per time point); *p < 0.05 versus sham controls (ANOVA and_post hoc_ Fisher’s PLSD tests).
Fig. 7.
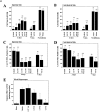
a, Quantitative analysis of effect of in vivo caspase-3 inhibitor treatment on hippocampal CA1 neuron survival (A, B), DNA fragmentation (C, D), and caspase-3-like activity after ischemia (E). A,B, Dose-dependent increase in CA1 neuron survival after ischemia by the caspase-3 inhibitor Z-DEVD-FMK, either injected before the induction of ischemia (vehicle or total dose of 0.5, 1.5, or 4.5 μg) or after ischemia (post-is with total dose of 4.5 μg). Cresyl violet staining and cell counting were performed either 3 or 7 d after ischemia. C, D, Dose-dependent decrease in the amount of cells with DNA fragmentation (TUNEL positive) in CA1 after ischemia by inhibiting caspase-3-like protease activity. No DNA fragmentation is present in CA1 in naive or sham-operated brains (data not shown). Sections through the same level of the dorsal hippocampus (bregma, −4.0 mm) are used for the analysis. Cell counting includes the entire CA1 sector at this level. E, Dose-dependent inhibition of caspase-3-like activity in the hippocampus by Z-DEVD-FMK. Vehicle (n = 4) or the peptide (total dose of 0.17, 0.5, 1.5, or 4.5 μg) was infused beginning 30 min before ischemia (n = 5 each dose). Caspase-3-like activity was measured in hippocampal cell extracts 48 hr after ischemia. All data are reported as mean ± SEM; *p < 0.01, and ** p < 0.001 (ANOVA and post hoc Fisher’s PLSD tests).b, Quantitative analysis of effect of in vivo ICE inhibitor Z-YVAD-FMK treatment on hippocampal CA1 neuron survival (A) and DNA fragmentation (B) 3 d after ischemia. No significant protection by Z-YVAD-FMK was detected. Injection Side, The hemisphere receiving inhibitor infusion; contralateral side, the hemisphere receiving no infusion. The_number_ in parentheses indicates the number of animals in that experimental group.
Fig. 7.
a, Quantitative analysis of effect of in vivo caspase-3 inhibitor treatment on hippocampal CA1 neuron survival (A, B), DNA fragmentation (C, D), and caspase-3-like activity after ischemia (E). A,B, Dose-dependent increase in CA1 neuron survival after ischemia by the caspase-3 inhibitor Z-DEVD-FMK, either injected before the induction of ischemia (vehicle or total dose of 0.5, 1.5, or 4.5 μg) or after ischemia (post-is with total dose of 4.5 μg). Cresyl violet staining and cell counting were performed either 3 or 7 d after ischemia. C, D, Dose-dependent decrease in the amount of cells with DNA fragmentation (TUNEL positive) in CA1 after ischemia by inhibiting caspase-3-like protease activity. No DNA fragmentation is present in CA1 in naive or sham-operated brains (data not shown). Sections through the same level of the dorsal hippocampus (bregma, −4.0 mm) are used for the analysis. Cell counting includes the entire CA1 sector at this level. E, Dose-dependent inhibition of caspase-3-like activity in the hippocampus by Z-DEVD-FMK. Vehicle (n = 4) or the peptide (total dose of 0.17, 0.5, 1.5, or 4.5 μg) was infused beginning 30 min before ischemia (n = 5 each dose). Caspase-3-like activity was measured in hippocampal cell extracts 48 hr after ischemia. All data are reported as mean ± SEM; *p < 0.01, and ** p < 0.001 (ANOVA and post hoc Fisher’s PLSD tests).b, Quantitative analysis of effect of in vivo ICE inhibitor Z-YVAD-FMK treatment on hippocampal CA1 neuron survival (A) and DNA fragmentation (B) 3 d after ischemia. No significant protection by Z-YVAD-FMK was detected. Injection Side, The hemisphere receiving inhibitor infusion; contralateral side, the hemisphere receiving no infusion. The_number_ in parentheses indicates the number of animals in that experimental group.
Fig. 8.
Effect of in vivo caspase-3 inhibition on CA1 neuron survival and DNA fragmentation.A, Low-power fields (40×) showing representative cresyl violet staining (a, c, e) and TUNEL (b, d, f) in the hippocampus 3 d after sham operation (a,b), after ischemia plus vehicle infusion (c, d), or after ischemia plus caspase-3 inhibitor infusion (total dose, 4.5 μg; e, f).Arrows in c and e mark cell death in the CA1 sector. Arrows in _d_and f mark DNA-fragmented (TUNEL-positive) cells in the CA1 sector. B, High-power fields (400×) showing representative cresyl violet staining (a,c, e, g) and TUNEL counterstained with cresyl violet (b, d,f, h) in the CA1 sector. Three days after sham operation (a, b), no cell death or DNA fragmentation is present in CA1; 3 d after ischemia plus vehicle infusion (c, d), the majority of CA1 neurons show pyknotic changes (c) and TUNEL labeling (d); 3 d after ischemia plus caspase-3 inhibitor infusion (e,f), many neurons show normal morphology (yellow arrowheads), and decreased amounts of neurons show pyknotic changes (e) or TUNEL labeling (red arrowheads; f); and 7 d after ischemia plus inhibitor infusion (g, h), both survival neurons (yellow arrowheads) and TUNEL-positive cells (red arrowheads; h) are present in the CA1. Scale bar, 50 μm.
Similar articles
- Cloning and characterization of rat caspase-9: implications for a role in mediating caspase-3 activation and hippocampal cell death after transient cerebral ischemia.
Cao G, Luo Y, Nagayama T, Pei W, Stetler RA, Graham SH, Chen J. Cao G, et al. J Cereb Blood Flow Metab. 2002 May;22(5):534-46. doi: 10.1097/00004647-200205000-00005. J Cereb Blood Flow Metab. 2002. PMID: 11973426 - Caspase-activated DNase/DNA fragmentation factor 40 mediates apoptotic DNA fragmentation in transient cerebral ischemia and in neuronal cultures.
Cao G, Pei W, Lan J, Stetler RA, Luo Y, Nagayama T, Graham SH, Yin XM, Simon RP, Chen J. Cao G, et al. J Neurosci. 2001 Jul 1;21(13):4678-90. doi: 10.1523/JNEUROSCI.21-13-04678.2001. J Neurosci. 2001. PMID: 11425895 Free PMC article. - Transient global forebrain ischemia induces a prolonged expression of the caspase-3 mRNA in rat hippocampal CA1 pyramidal neurons.
Ni B, Wu X, Su Y, Stephenson D, Smalstig EB, Clemens J, Paul SM. Ni B, et al. J Cereb Blood Flow Metab. 1998 Mar;18(3):248-56. doi: 10.1097/00004647-199803000-00003. J Cereb Blood Flow Metab. 1998. PMID: 9498841 - Caspase function in neuronal death: delineation of the role of caspases in ischemia.
Prunell GF, Arboleda VA, Troy CM. Prunell GF, et al. Curr Drug Targets CNS Neurol Disord. 2005 Feb;4(1):51-61. doi: 10.2174/1568007053005082. Curr Drug Targets CNS Neurol Disord. 2005. PMID: 15723613 Review. - Implication of cysteine proteases calpain, cathepsin and caspase in ischemic neuronal death of primates.
Yamashima T. Yamashima T. Prog Neurobiol. 2000 Oct;62(3):273-95. doi: 10.1016/s0301-0082(00)00006-x. Prog Neurobiol. 2000. PMID: 10840150 Review.
Cited by
- Cyclooxygenase-2-specific inhibitor improves functional outcomes, provides neuroprotection, and reduces inflammation in a rat model of traumatic brain injury.
Gopez JJ, Yue H, Vasudevan R, Malik AS, Fogelsanger LN, Lewis S, Panikashvili D, Shohami E, Jansen SA, Narayan RK, Strauss KI. Gopez JJ, et al. Neurosurgery. 2005 Mar;56(3):590-604. doi: 10.1227/01.neu.0000154060.14900.8f. Neurosurgery. 2005. PMID: 15730585 Free PMC article. - Poly(ADP-ribose) polymerase (PARP)-1-independent apoptosis-inducing factor (AIF) release and cell death are induced by eleostearic acid and blocked by alpha-tocopherol and MEK inhibition.
Kondo K, Obitsu S, Ohta S, Matsunami K, Otsuka H, Teshima R. Kondo K, et al. J Biol Chem. 2010 Apr 23;285(17):13079-91. doi: 10.1074/jbc.M109.044206. Epub 2010 Feb 22. J Biol Chem. 2010. PMID: 20177052 Free PMC article. - Enhanced Delivery of Erythropoietin Across the Blood-Brain Barrier for Neuroprotection against Ischemic Neuronal Injury.
Zhang F, Xing J, Liou AK, Wang S, Gan Y, Luo Y, Ji X, Stetler RA, Chen J, Cao G. Zhang F, et al. Transl Stroke Res. 2010 Jun;1(2):113-21. doi: 10.1007/s12975-010-0019-3. Transl Stroke Res. 2010. PMID: 20577577 Free PMC article. - Neuroprotective and disease-modifying effects of the ketogenic diet.
Gasior M, Rogawski MA, Hartman AL. Gasior M, et al. Behav Pharmacol. 2006 Sep;17(5-6):431-9. doi: 10.1097/00008877-200609000-00009. Behav Pharmacol. 2006. PMID: 16940764 Free PMC article. Review. - Neurodegeneration in Lurcher mice occurs via multiple cell death pathways.
Doughty ML, De Jager PL, Korsmeyer SJ, Heintz N. Doughty ML, et al. J Neurosci. 2000 May 15;20(10):3687-94. doi: 10.1523/JNEUROSCI.20-10-03687.2000. J Neurosci. 2000. PMID: 10804210 Free PMC article.
References
- Alnemri ES, Livingston DJ, Nicholson DW, Salvesen G, Thronberry NA, Wong WW. Human ICE/CED-3 protease nomenclature. Cell. 1996;87:171. - PubMed
- Althaus FR, Richter C. ADP-ribosylation of proteins. Enzymology and biological significance. Mol Biol Biochem Biophys. 1987;37:1–237. - PubMed
- Bredesen DE. Neural apoptosis. Ann Neurol. 1995;38:839–851. - PubMed
- Charriaut-Marlangue C, Margaill I, Represa A, Popovici T, Plotkine M, Ben-Ari Y. Apoptosis and necrosis after reversible focal ischemia: an in situ DNA fragmentation analysis. J Cereb Blood Flow Metab. 1996;16:186–194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS024728/NS/NINDS NIH HHS/United States
- NS 35965/NS/NINDS NIH HHS/United States
- NS 24728/NS/NINDS NIH HHS/United States
- P01 NS035965/NS/NINDS NIH HHS/United States
- NS 36736/NS/NINDS NIH HHS/United States
- R01 NS036736/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous