Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons - PubMed (original) (raw)
. 1998 Jul 1;18(13):4929-37.
doi: 10.1523/JNEUROSCI.18-13-04929.1998.
M C Nishimura, M P Armanini, L C Wang, K T Poulsen, C Rosenblad, D Kirik, B Moffat, L Simmons, E Johnson Jr, J Milbrandt, A Rosenthal, A Bjorklund, R A Vandlen, M A Hynes, H S Phillips
Affiliations
- PMID: 9634558
- PMCID: PMC6792569
- DOI: 10.1523/JNEUROSCI.18-13-04929.1998
Neurturin exerts potent actions on survival and function of midbrain dopaminergic neurons
B A Horger et al. J Neurosci. 1998.
Abstract
Glial cell line-derived neurotrophic factor (GDNF) exhibits potent effects on survival and function of midbrain dopaminergic (DA) neurons in a variety of models. Although other growth factors expressed in the vicinity of developing DA neurons have been reported to support survival of DA neurons in vitro, to date none of these factors duplicate the potent and selective actions of GDNF in vivo. We report here that neurturin (NTN), a homolog of GDNF, is expressed in the nigrostriatal system, and that NTN exerts potent effects on survival and function of midbrain DA neurons. Our findings indicate that NTN mRNA is sequentially expressed in the ventral midbrain and striatum during development and that NTN exhibits survival-promoting actions on both developing and mature DA neurons. In vitro, NTN supports survival of embryonic DA neurons, and in vivo, direct injection of NTN into the substantia nigra protects mature DA neurons from cell death induced by 6-OHDA. Furthermore, administration of NTN into the striatum of intact adult animals induces behavioral and biochemical changes associated with functional upregulation of nigral DA neurons. The similarity in potency and efficacy of NTN and GDNF on DA neurons in several paradigms stands in contrast to the differential distribution of the receptor components GDNF Family Receptor alpha1 (GFRalpha1) and GFRalpha2 within the ventral mesencephalon. These results suggest that NTN is an endogenous trophic factor for midbrain DA neurons and point to the possibility that GDNF and NTN may exert redundant trophic influences on nigral DA neurons acting via a receptor complex that includes GFRalpha1.
Figures
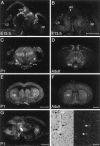
Fig. 1.
NTN mRNA appears sequentially in the ventral midbrain (vm) and caudate putamen (cp) of the developing mouse brain. Sections of E13.5 brain hybridized with antisense probe to NTN (A) display strong hybridization in the ventral midbrain (vm), whereas no hybridization is seen in the developing caudate putamen (cp) above the background seen with sense strand control probe (B). At P1, (C, E, G) as well as in the adult brain (D, F) hybridization is seen in both the ventral midbrain and caudate putamen. The signal in the adult caudate (F, H) is associated with cells displaying a nuclear morphology characteristic of neurons. Scale bars:B, E, F, 1 μm (these apply to A, C,D, respectively); G, 1 μm;H, 0.1 μm.
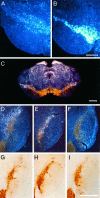
Fig. 2.
mRNA for GFRα2 is localized in the vicinity of nigral DA neurons. GFRα2 mRNA (A) expression is much more diffuse and weak than GFRα1 (B) in the region of the pars compacta of the substantia nigra. Colocalization of TH staining (brown) and in situ_hybridization for mRNA (white silver grains) for GFRα2 (C) reveals that the majority of GFRα2 expression in the adult ventral midbrain is not produced by DA neurons but by cells residing nearby. In particular, a band of GFRα2-expressing cells is seen in a zone that is dorsolateral to the pars compacta. D–F, Dark-field images of the same sections shown under bright-field illumination in G–I. All sections were immunostained for TH (brown).In situ hybridizations were performed for GFRα2 (D, G), GFRα1 (E, H), and sense strand control probe (F, I). Marginally more silver grains are seen for GFRα2 hybridization over TH+ cells (D, G) than in sections hybridized with a sense strand control probe to GFRα1 (F, I). Scale bars: B, 0.5 μm (applies to A);C, I, 1 μm (I applies to_D–I).
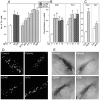
Fig. 3.
NTN promotes survival of midbrain DA neurons_in vitro_ and in vivo. Values in_A–C_ represent mean ± SEM; *p< 0.05; **p < 0.0001 (vs control).A, NTN promotes survival of TH+ cells in cultures of E14 rat ventral mesencephalon. Survival responses to maximally effective concentration of NTN are very similar to those seen with optimal doses of GDNF. B, Single bolus injection of NTN can provide partial protection of FG+ or TH+ nigral cells after intrastriatal 6-OHDA administration. Single intranigral injections of 1 or 10 μg of NTN or GDNF, administered 1 week after the toxic insult, produce comparable sparing of FG+ cells after 6-OHDA. A single intranigral injection of 10 μg of NTN can produce sparing of TH+ cells, whereas 1 μg is not effective. Note the comparable effects of similar doses of GDNF and NTN at both doses on TH+ cells. C, Repeated administration of NTN rescued all FG+ cells, but because a high proportion of the FG+ cells were TH-negative, the TH+ cell number was not significantly increased in the NTN-treated rats. In a series of double-labeled sections, the percentage of FG+ cells that expressed TH was reduced not only in the NTN-treated nigra but also in the controls treated with vehicle alone (from 80 ± 3% on the nontreated intact side to 52 ± 6% on the treated side), indicating a potential deleterious effect of repeated administration of the acidic, hypotonic vehicle (data not shown). D, Appearance of FG+ nigral cells after neurotoxic insult and rescue with GDNF or NTN. The_top left panel_ represents a control substantia nigra (contralateral to 6-OHDA administration), whereas the top right panel depicts a vehicle-treated substantia nigra 4 weeks after ipsilateral intrastriatal 6-OHDA treatment. The lesioned nigra shows very few surviving neurons but many small cells of microglial morphology. The bottom panels demonstrate that a single bolus injection of 10 μg of either NTN or GDNF protects the survival of many fluorogold-labeled neurons at 4 weeks after 6-OHDA administration. E, Appearance of TH+ cells after neurotoxic insult and rescue with GDNF or NTN. _Top left_and top right panels depict substantia nigra 4 weeks after 6-OHDA to the contralateral (left) or ipsilateral (right) striatum. The brain in the top right panel was treated by injection of vehicle into the pars compacta of the substantia nigra. Partial protection of TH-expressing cells is seen after a single bolus injection of 10 μg of either NTN or GDNF into the pars compacta.
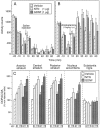
Fig. 4.
Intrastriatal injection of NTN or GDNF augments amphetamine-induced locomotor activity and increases striatal DA utilization. A, B, Injections of NTN or GDNF (1 μg) into the right hemisphere do not significantly alter spontaneous open-field locomotor activity but do augment amphetamine-induced locomotor activity. A, B, Mean ± SEM number of interrupted photocell beams 30 min before and 60 min after saline (A) or amphetamine (1 mg/kg, i.p.) (B) administration. _Asterisks_indicate significant differences from vehicle-injected controls (p < 0.05). C, Mean ± SEM ratio of the metabolite DOPAC to DA in each brain region sampled 1 week after unilateral administration of NTN, GDNF, or vehicle in the right striatum. Data are depicted as percent of the noninjected (intact) hemisphere. Significant increases from vehicle-injected controls as determined by Fisher’s post hoc analyses are indicated by an asterisk(p < 0.05). Except for the 1 and 10 μg doses of NTN in the posterior striatum, individual doses within the same treatment group were significantly different from each other (p < 0.05). Subsequent comparisons revealed a significantly greater effect of the 0.1 μg dose of NTN relative to the same dose of GDNF at all three striatal sites. In the nucleus accumbens, DA utilization was increased by both 1 and 10 μg doses of GDNF, whereas only the highest dose of NTN reached significance.
Similar articles
- Protection and regeneration of nigral dopaminergic neurons by neurturin or GDNF in a partial lesion model of Parkinson's disease after administration into the striatum or the lateral ventricle.
Rosenblad C, Kirik D, Devaux B, Moffat B, Phillips HS, Björklund A. Rosenblad C, et al. Eur J Neurosci. 1999 May;11(5):1554-66. doi: 10.1046/j.1460-9568.1999.00566.x. Eur J Neurosci. 1999. PMID: 10215908 - Differential effects of glial cell line-derived neurotrophic factor and neurturin on developing and adult substantia nigra dopaminergic neurons.
Akerud P, Alberch J, Eketjäll S, Wagner J, Arenas E. Akerud P, et al. J Neurochem. 1999 Jul;73(1):70-8. doi: 10.1046/j.1471-4159.1999.0730070.x. J Neurochem. 1999. PMID: 10386956 - Other neurotrophic factors: glial cell line-derived neurotrophic factor (GDNF).
Saarma M, Sariola H. Saarma M, et al. Microsc Res Tech. 1999 May 15-Jun 1;45(4-5):292-302. doi: 10.1002/(SICI)1097-0029(19990515/01)45:4/5<292::AID-JEMT13>3.0.CO;2-8. Microsc Res Tech. 1999. PMID: 10383122 Review. - Adenoviral vector-mediated delivery of glial cell line-derived neurotrophic factor provides neuroprotection in the aged parkinsonian rat.
Connor B. Connor B. Clin Exp Pharmacol Physiol. 2001 Nov;28(11):896-900. doi: 10.1046/j.1440-1681.2001.03544.x. Clin Exp Pharmacol Physiol. 2001. PMID: 11703392 Review.
Cited by
- Oxytocin signaling is necessary for synaptic maturation of adult-born neurons.
Pekarek BT, Kochukov M, Lozzi B, Wu T, Hunt PJ, Tepe B, Hanson Moss E, Tantry EK, Swanson JL, Dooling SW, Patel M, Belfort BDW, Romero JM, Bao S, Hill MC, Arenkiel BR. Pekarek BT, et al. Genes Dev. 2022 Nov-Dec 1;36(21-24):1100-1118. doi: 10.1101/gad.349930.122. Epub 2022 Dec 8. Genes Dev. 2022. PMID: 36617877 Free PMC article. - Treatment of Parkinson's disease with trophic factors.
Peterson AL, Nutt JG. Peterson AL, et al. Neurotherapeutics. 2008 Apr;5(2):270-80. doi: 10.1016/j.nurt.2008.02.003. Neurotherapeutics. 2008. PMID: 18394569 Free PMC article. Review. - Revisiting the Role of Neurotrophic Factors in Inflammation.
Morel L, Domingues O, Zimmer J, Michel T. Morel L, et al. Cells. 2020 Apr 2;9(4):865. doi: 10.3390/cells9040865. Cells. 2020. PMID: 32252363 Free PMC article. Review. - The current status of gene therapy for Parkinson's disease.
Muramatsu S. Muramatsu S. Ann Neurosci. 2010 Apr;17(2):92-5. doi: 10.5214/ans.0972-7531.1017209. Ann Neurosci. 2010. PMID: 25205879 Free PMC article. Review. - Anatomical basis of glial cell line-derived neurotrophic factor expression in the striatum and related basal ganglia during postnatal development of the rat.
Oo TF, Ries V, Cho J, Kholodilov N, Burke RE. Oo TF, et al. J Comp Neurol. 2005 Mar 28;484(1):57-67. doi: 10.1002/cne.20463. J Comp Neurol. 2005. PMID: 15717300 Free PMC article.
References
- Baloh RH, Tansey MG, Golden JP, Creedon DJ, Heuckeroth RO, Keck CL, Zimonjic DB, Popescu NC, Johnson EM, Jr, Milbrandt J. TrnR2, a novel receptor that mediates neurturin and GDNF signaling through Ret. Neuron. 1997;18:793–802. - PubMed
- Beck KD, Valverde J, Alexi T, Poulsen K, Moffat B, Vandlen RA, Rosenthal A, Hefti F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373:339–341. - PubMed
- Bowenkamp KE, Hoffman AF, Gerhardt GA, Henry MA, Biddle PT, Hoffer BJ, Granholm AC. Glial cell line-derived neurotrophic factor supports survival of injured midbrain dopaminergic neurons. J Comp Neurol. 1995;355:479–489. - PubMed
- Buj-Bello A, Adu J, Piñón P, Horton A, Thompson J, Rosenthal A, Chinchetru M, Buchman VL, Davies AM. Neurturin responsiveness requires a GPI-linked receptor and the Ret receptor tyrosine kinase. Nature. 1997;387:721–724. - PubMed
- Durbec PL, Larsson-Blomberg LB, Schuchardt A, Costantini F, Pachnis V. Common origin and developmental dependence on c-ret of subsets of enteric and sympathetic neuroblasts. Development. 1996;122:349–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials