Malignant transformation of early lymphoid progenitors in mice expressing an activated Blk tyrosine kinase - PubMed (original) (raw)
Malignant transformation of early lymphoid progenitors in mice expressing an activated Blk tyrosine kinase
S N Malek et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The intracellular signals governing cellular proliferation and developmental progression during lymphocyte development are incompletely understood. The tyrosine kinase Blk is expressed preferentially in the B lineage, but its function in B cell development has been largely unexplored. We have generated transgenic mice expressing constitutively active Blk [Blk(Y495F)] in the B and T lymphoid compartments. Expression of Blk(Y495F) in the B lineage at levels similar to that of endogenous Blk induced B lymphoid tumors of limited clonality, whose phenotypes are characteristic of B cell progenitors at the proB/preB-I to preB-II transition. Expression of constitutively active Blk in the T lineage resulted in the appearance of clonal, thymic lymphomas composed of intermediate single positive cells. Taken together, these results indicate that specific B and T cell progenitor subsets are preferentially susceptible to transformation by Blk(Y495F) and suggest a role for Blk in the control of proliferation during B cell development.
Figures
Figure 1
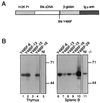
Expression of mutant Blk proteins in transgenic mice. (A) Organization of Blk transgenes. The H2K promoter, Blk cDNA, β-globin splice donor and acceptor sequences, and the Ig heavy chain intronic enhancer are indicated by rectangles. The Y495F mutation is indicated by a vertical line. (B) Expression of constitutively active mutant Blk protein in transgenic mice. Thymocytes (lanes 1–5) or splenic B cells (lanes 6–11) from transgenic mice (lanes 1–4; lanes 6–10) and nontransgenic control animals (lanes 5 and 11) were assayed for Blk by immunoblotting. Individual transgenic mice from which samples were derived are identified across the top. Spleens from Blk(Y495F)-6, -13, -15, and -16 were macroscopically normal; tumor was evident in the spleen from founder animal Blk(Y495F)-29. The apparent sizes (in kDa) and positions of molecular mass standards are indicated at right.
Figure 2
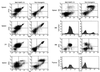
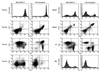
Expansion and dissemination of B220+CD43+ B cell progenitors in the Blk(Y495F)-15 transgenic line. Single-cell suspensions were prepared from spleen, lymph nodes, thymus, and bone marrow from lymphomatous Blk(Y495)-15 transgenic mice or from age-matched, nontransgenic littermates. Cells were stained, singly or in combination, with PE- (y axis) or FITC- (x axis) conjugated antibodies to CD19, B220, CD22, IgM, IgD, and CD43 as indicated. The founder, five transgenic progeny, and four normal mice were examined; representative results are presented.
Figure 3
Expansion and dissemination of CD3−CD4−CD8+ T cell progenitors in the Blk(Y495F)-6 transgenic line. Single-cell suspensions were made from thymus, spleen, lymph nodes, and bone marrow from thymomatous Blk(Y495)-6 transgenic mice or from age-matched, nontransgenic littermates. Cells were stained, singly or in combination, with PE- (y axis) and FITC- (x axis) conjugated antibodies to Thy1.2, CD3, αβ TCR, CD8, CD4, CD44, CD25, CD19, and B220 as indicated. The founder, four transgenic progeny, and three normal mice were examined; representative results are presented.
Figure 4
Clonality of lymphoid tumors in Blk(Y495F) transgenic mice. (A) Analysis of TCR-β rearrangements. Genomic DNA was extracted from thymus (T), spleen (S), lymph node (LN), or liver (Li) of transgenic mice (lanes 1–13) or nontransgenic littermates (lanes 14–16). The following individual transgenic mice were assayed: Blk(Y495F)-6 founder (lanes 1–3); Blk(Y495F)-6.15 (lanes 4 and 5); the Blk(Y495F)-16 founder (lanes 6–8); Blk(Y495F)-16.32 (lane 9); Blk(Y495F)-16.34 (lanes 10 and 11); Blk(Y495F)-60 founder (lanes 12 and 13). DNA was digested with _Bgl_II and assayed for rearrangement by Southern hybridization using a probe derived from the Jβ-Cβ1 intron. (B) Analysis of Ig heavy chain rearrangements. Genomic DNA was extracted from lymphoid organs of Blk(Y495F)-15.4 (lanes 3, 4, 10, and 11), Blk(Y495F)-15.15 (lanes 5–7 and 12–14), or nontransgenic littermates (lanes 1, 2, 8, and 9). DJH and VHDJH rearrangements were amplified by PCR as described in ref. ; products were fractionated by agarose gel electrophoresis and detected with ethidium bromide. Lanes 1–7, assays for DJH rearrangements; lanes 8–14, assays for VHDJH rearrangements. The sizes of DNA standards, in bp, are indicated at left. (C) Analysis of junctional heterogeneity in Ig heavy chain rearrangements. DJH3- and VHDJH3-containing PCR fragments from Blk(Y495F)-15.15 (lanes 2–4 and 6–8) or normal mice (lanes 1 and 5) were purified from an agarose gel and reamplified by using a radiolabeled J3 primer and D or VH primers as described (28). Products were fractionated by electrophoresis through a 5% polyacrylamide-urea gel and detected using a PhosphorImager. Sizes of DNA standards, in bp, are indicated at right.
Figure 5
Expression of Blk(Y495F) protein at sequential stages of lymphoid development. Bone marrow cells were obtained from macroscopically tumor-free Blk(Y495F)-15 transgenic mice (lanes 1–3) or nontransgenic littermates (lanes 4–6) at 4–6 weeks of age. B220+CD43+ (lanes 3 and 6) and B220+CD43− (lanes 2 and 5) populations were purified by FACS. Protein corresponding to 105 cells from unfractionated bone marrow (lanes 1 and 4) or sorted populations was assayed for Blk by immunoblotting. Thymocytes were prepared from macroscopically tumor-free Blk(Y495F)-6 (lanes 7–10) or Blk(Y495F)-13 (lanes 11–14) transgenic mice at 4–6 weeks of age. CD4−CD8− (lanes 8 and 12), CD4+CD8+ (lanes 9 and 13), and CD4+CD8− (lanes 10 and 14) populations were purified by FACS. Protein corresponding to 106 cells from unfractionated thymus (lanes 7 and 11) or sorted populations was assayed for Blk by immunoblotting. The apparent sizes (in kDa) and positions of molecular mass standards are indicated.
Figure 6
Protein–tyrosine phosphorylation in Blk(Y495F)-induced lymphoid tumors. (A) Lymph nodes (lanes 1, 3, 4, and 6), spleens (lanes 2, 5, and 7), or thymuses (lanes 8–10) from Blk(Y495F) transgenic mice (lanes 1–6; lanes 8 and 9) or nontransgenic littermates (lanes 7 and 10) were dispersed and lysed. Phosphotyrosine-containing proteins in 106 cell equivalents were detected by immunoblotting. The following transgenic mice were examined: Blk(Y495F)-15.4 (lanes 1 and 2); Blk(Y495F)-15.15 (lane 3); Blk(Y495F)-15.3 (lanes 4 and 5); Blk(Y495F)-29F (lane 6); Blk(Y495F)-16F (lane 8); and Blk(Y495F)-13.11 (lane 9). (B and C) B lymphoid tumor-bearing lymph nodes (B, lanes 1 and 3) and T lymphoid tumor-bearing thymuses (C, lanes 1, 3, and 5) from Blk(Y495F) transgenic mice were dispersed; splenocytes (B, lanes 2 and 4) and thymocytes (C, lanes 2, 4, and 6) from nontransgenic littermates were prepared in parallel. PLC-γ, PI-3K, and ZAP-70 were immunoprecipitated from 5 × 107 cell equivalents of cell lysate, and phosphotyrosine was detected by immunoblotting as in A. The apparent sizes (in kDa) and positions of molecular mass standards are indicated at left. Arrowheads indicate individual proteins as described in the text.
Similar articles
- Dysfunctional BLK in common variable immunodeficiency perturbs B-cell proliferation and ability to elicit antigen-specific CD4+ T-cell help.
Compeer EB, Janssen W, van Royen-Kerkhof A, van Gijn M, van Montfrans JM, Boes M. Compeer EB, et al. Oncotarget. 2015 May 10;6(13):10759-71. doi: 10.18632/oncotarget.3577. Oncotarget. 2015. PMID: 25926555 Free PMC article. - The B-cell-specific Src-family kinase Blk is dispensable for B-cell development and activation.
Texido G, Su IH, Mecklenbräuker I, Saijo K, Malek SN, Desiderio S, Rajewsky K, Tarakhovsky A. Texido G, et al. Mol Cell Biol. 2000 Feb;20(4):1227-33. doi: 10.1128/MCB.20.4.1227-1233.2000. Mol Cell Biol. 2000. PMID: 10648608 Free PMC article. - Mimicry of pre-B cell receptor signaling by activation of the tyrosine kinase Blk.
Tretter T, Ross AE, Dordai DI, Desiderio S. Tretter T, et al. J Exp Med. 2003 Dec 15;198(12):1863-73. doi: 10.1084/jem.20030729. Epub 2003 Dec 8. J Exp Med. 2003. PMID: 14662906 Free PMC article. - Specific expression of a tyrosine kinase gene, blk, in B lymphoid cells.
Dymecki SM, Niederhuber JE, Desiderio SV. Dymecki SM, et al. Science. 1990 Jan 19;247(4940):332-6. doi: 10.1126/science.2404338. Science. 1990. PMID: 2404338 - Fidelity and infidelity in commitment to B-lymphocyte lineage development.
Rolink AG, Schaniel C, Busslinger M, Nutt SL, Melchers F. Rolink AG, et al. Immunol Rev. 2000 Jun;175:104-11. Immunol Rev. 2000. PMID: 10933595 Review.
Cited by
- ADAM10 overexpression shifts lympho- and myelopoiesis by dysregulating site 2/site 3 cleavage products of Notch.
Gibb DR, Saleem SJ, Kang DJ, Subler MA, Conrad DH. Gibb DR, et al. J Immunol. 2011 Apr 1;186(7):4244-52. doi: 10.4049/jimmunol.1003318. Epub 2011 Mar 2. J Immunol. 2011. PMID: 21368228 Free PMC article. - Constitutive activation of the SRC family kinase Hck results in spontaneous pulmonary inflammation and an enhanced innate immune response.
Ernst M, Inglese M, Scholz GM, Harder KW, Clay FJ, Bozinovski S, Waring P, Darwiche R, Kay T, Sly P, Collins R, Turner D, Hibbs ML, Anderson GP, Dunn AR. Ernst M, et al. J Exp Med. 2002 Sep 2;196(5):589-604. doi: 10.1084/jem.20020873. J Exp Med. 2002. PMID: 12208875 Free PMC article. - Cyclooxygenase inhibitors block uterine tumorigenesis in HMGA1a transgenic mice and human xenografts.
Di Cello F, Hillion J, Kowalski J, Ronnett BM, Aderinto A, Huso DL, Resar LM. Di Cello F, et al. Mol Cancer Ther. 2008 Jul;7(7):2090-5. doi: 10.1158/1535-7163.MCT-07-2282. Mol Cancer Ther. 2008. PMID: 18645019 Free PMC article. - Epstein-Barr virus latent membrane protein 2A preferentially signals through the Src family kinase Lyn.
Rovedo M, Longnecker R. Rovedo M, et al. J Virol. 2008 Sep;82(17):8520-8. doi: 10.1128/JVI.00843-08. Epub 2008 Jun 25. J Virol. 2008. PMID: 18579586 Free PMC article. - Smad3-dependent nuclear translocation of beta-catenin is required for TGF-beta1-induced proliferation of bone marrow-derived adult human mesenchymal stem cells.
Jian H, Shen X, Liu I, Semenov M, He X, Wang XF. Jian H, et al. Genes Dev. 2006 Mar 15;20(6):666-74. doi: 10.1101/gad.1388806. Genes Dev. 2006. PMID: 16543220 Free PMC article.
References
- Lin W-C, Desiderio S. Immunol Today. 1995;16:279–289. - PubMed
- Shinkai Y, Koyasu S, Nakayama K, Murphy K M, Loh D Y, Reinherz E L, Alt F W. Science. 1993;259:822–825. - PubMed
- Karasuyama H, Rolink A, Shinkai Y, Young F, Alt F W, Melchers F. Cell. 1994;77:133–143. - PubMed
- Rolink A, Grawunder U, Winkler T H, Karasuyama H, Melchers F. Int Immunol. 1994;6:1257–1264. - PubMed
- Spanopoulou E, Roman C A J, Corcoran L M, Schlissel M S, Silver D P, Nemazee D, Nussenzweig M C, Shinton S A, Hardy R R, Baltimore D. Genes Dev. 1994;8:1030–1042. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous