Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle - PubMed (original) (raw)
Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle
F Verde et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The molecular mechanisms that coordinate cell morphogenesis with the cell cycle remain largely unknown. We have investigated this process in fission yeast where changes in polarized cell growth are coupled with cell cycle progression. The orb6 gene is required during interphase to maintain cell polarity and encodes a serine/threonine protein kinase, belonging to the myotonic dystrophy kinase/cot1/warts family. A decrease in Orb6 protein levels leads to loss of polarized cell shape and to mitotic advance, whereas an increase in Orb6 levels maintains polarized growth and delays mitosis by affecting the p34(cdc2) mitotic kinase. Thus the Orb6 protein kinase coordinates maintenance of cell polarity during interphase with the onset of mitosis. orb6 interacts genetically with orb2, which encodes the Pak1/Shk1 protein kinase, a component of the Ras1 and Cdc42-dependent signaling pathway. Our results suggest that Orb6 may act downstream of Pak1/Shk1, forming part of a pathway coordinating cell morphogenesis with progression through the cell cycle.
Figures
Figure 1
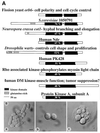
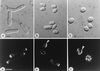
Cloning of orb6+. (a) leu1–32, pRep3X. (b) orb6–25 leu1–32, pRep3X. (c) orb6–25 leu1–32, pRep3X orb6+. Cells were exponentially grown at 25°C for at least eight generations, then shifted at 36°C for 5 hr.
Figure 2
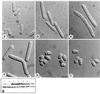
The orb6+ gene. (A) Sequence comparison of Orb6 and homologous proteins. An homology search using EMBL and GenBank databases identified S. cerevisiae 1050791, Neurospora crassa cot1 (14), human Ndr (23), Drosophila warts (15, 16), human PK428, Rho-associated kinase (–19), and human DMPK (20). (B) orb6+ gene deletion. Spores were plated on yeast extract plates and allowed to germinate for 16 hr at 32°C. (a) ade- leu1–32 ura4-D18 microcolony. (b) orb6_∷_ura4+ ade- leu1–32 ura4-D18 microcolony. (c) orb6_∷_ura4+ ade- leu1–32 ura4-D18 microcolony after 24 hr at 32°C. (Bar = 5 μm.)
Figure 3
Orb6+ gene dosage effects. orb6_∷_ura4+ ade- leu1–32 ura4-D18 cells containing integrated HA-tagged orb6+ were grown exponentially for at least eight generations at 32°C, then thiamine was added to the culture. (a and d) Cells grown in the absence of thiamine. (b and e) 7.5 hr after thiamine addition. (c and f) 12 hr after thiamine addition. Cells were photographed immediately (a_–_c) or fixed and stained for actin (d_–_f). (Bar, a_–_c = 5 μm.; d_–_f = 10 μm.)
Figure 4
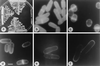
Effects of orb6 overexpression. (a_–_c) Overexpression of HA-tagged orb6+ in a leu1–32 strain (experiment 1 in Table 1). (a) leu1–23 pRep3X. (b) leu1–23 cells, carrying integrated HA-tagged orb6+, were grown exponentially for at least eight generations at 32°C in the absence of thiamine. (c) leu1–32 cells, carrying HA-tagged orb6+ on the multicopy plasmid pRep3X, were grown in the absence of thiamine for 18 hr at 32°C. (d_–_f) Overexpression of HA-tagged orb6+ in a wee1–50 mutant strain. Cells were grown exponentially at 25°C in the presence of thiamine for at least eight generations; thiamine was washed away and cells were grown in the absence of thiamine at 25°C for 16 hr, then shifted at 36°C for 5 hr. (d) leu1–32, pRep3X orb6HA. (e) wee1–50 leu1–32 pRep3X. (f) wee1–50 leu1–32 pRep3X orb6HA. (g) Western blot comparing the levels of expression of orb6-HA in the experiments described above, as compared with the control tubulin: lane 1, leu1–32 with an integrated copy of orb6-HA; lane 2, leu1–23 pRep3X; lane3, leu1–32 pRep3x orb6 HA; lane 4, leu1–32 pRep3x orb6 K122A HA; lane 5, wee1–50 leu1–32 pRep3x; lane 6, wee1–50 leu1–32 pRep3x orb6-HA. (Bar = 5 μm.)
Figure 5
Overexpression of Wee1 in orb6 mutants and actin localization in orb6–25 and wee1–50 mutants. (a_–_d) Overexpression of Wee1 in orb6 mutants. (a) _leu1–32_h− pRep3x. (b) _orb6–25 leu1–32_h− pRep3x. (c) _leu1–32_h− pRep3x wee1+. (d) _orb6–25 leu1–32_h− pRep3x wee1+. (e) _wee1–50_h−. Cells were grown exponentially at 25°C in the presence of thiamine for at least eight generations; thiamine was washed away, and cells were grown in the absence of thiamine at 25°C for 16 hr, then shifted at 36°C for 5 hr. (f_–_h) Actin localization in orb6–25 and wee1–50 mutants. (f) _leu1–32_h−. (g) _orb6–25 leu1–32_h−. (h) _wee1–50_h−. (Bar = 5 μm.)
Figure 6
orb6+ gene product intracellular localization in the cell cycle. (a, b, e, and f) orb6_∷_ura4+ ade- leu1–32 ura4-D18 cells containing integrated HA-tagged orb6+ were grown exponentially at 32°C in the absence of thiamine, then fixed and stained with an antibody recognizing the HA tag (a and b) and with 4′,6-diamidino-2-phenylindole (e and f). (g) Control staining of orb6_∷_ura4+ ade- leu1–32 ura4-D18 cells containing integrated untagged orb6+. (c) cdc25–22 leu1–32 Rep3X orb6 HA cells were grown exponentially at 25°C in the presence of thiamine for at least eight generations; thiamine was washed away and cells were grown in the absence of thiamine at 25°C for 16 hr, then shifted at 36°C for 4 hr. (d) orb2–34 leu1–32 pRep3X orb6 HA at 32°C; cells were stained 14 hr after thiamine depletion, which is about 2 hr after derepression of the nmt1 promoter. (h) orb2–34 leu1–32 stained for actin at 32°C. (Bar = 10 μm.)
Figure 7
Cloning of the orb2+ gene. (a) top, _ade6-M210 leu1–32_h−; left, _orb6–25 ade6-M210 leu1–32_h−; bottom, _orb2–34 ade6-M210 leu1–32_h−; right, orb2–34 orb6–25 ade6-M210 leu1–32 h−. Cells were grown at 32°C. (b) _ade6-M210 leu1–32_h−, Rep3X. (c) _orb2–34 ade6-M210 leu1–32_h−, pRep3X. (d) _orb2–34 ade6-M210 leu1–32_h− pDB248′-8 (genomic fragment). (e) _orb2–34 ade6-M210 leu1–32_h− pRep3X pak1/shk1+. (f) _orb2–34 ade6-M210 leu1–32_h− pRep3X orb6+. Cells were grown exponentially at 25°C in the presence of thiamine for at least eight generations; thiamine was washed away and cells were grown in the absence of thiamine at 25°C for 16 hr, then shifted at 36°C for 6 hr. (Bar = 5 μm.)
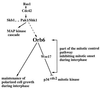
Figure 8
Orb6 kinase is proposed to be part of a regulatory cascade contributing to the mitotic control and responding to the Ras1 and Cdc42-dependent morphological control pathway via the Pak1/Shk1 kinase. Orb6 kinase regulates independently cell morphogenesis and the cell cycle, mediating its effect on p34cdc2 kinase directly through the Wee1 protein kinase or through some other mechanism dependent on p34cdc2 tyrosine phosphorylation.
Similar articles
- The conserved NDR kinase Orb6 controls polarized cell growth by spatial regulation of the small GTPase Cdc42.
Das M, Wiley DJ, Chen X, Shah K, Verde F. Das M, et al. Curr Biol. 2009 Aug 11;19(15):1314-9. doi: 10.1016/j.cub.2009.06.057. Epub 2009 Jul 30. Curr Biol. 2009. PMID: 19646873 - Shk1, a homolog of the Saccharomyces cerevisiae Ste20 and mammalian p65PAK protein kinases, is a component of a Ras/Cdc42 signaling module in the fission yeast Schizosaccharomyces pombe.
Marcus S, Polverino A, Chang E, Robbins D, Cobb MH, Wigler MH. Marcus S, et al. Proc Natl Acad Sci U S A. 1995 Jun 20;92(13):6180-4. doi: 10.1073/pnas.92.13.6180. Proc Natl Acad Sci U S A. 1995. PMID: 7597098 Free PMC article. - Fission Yeast NDR/LATS Kinase Orb6 Regulates Exocytosis via Phosphorylation of the Exocyst Complex.
Tay YD, Leda M, Spanos C, Rappsilber J, Goryachev AB, Sawin KE. Tay YD, et al. Cell Rep. 2019 Feb 5;26(6):1654-1667.e7. doi: 10.1016/j.celrep.2019.01.027. Cell Rep. 2019. PMID: 30726745 Free PMC article. - Cloning and characterization of shk2, a gene encoding a novel p21-activated protein kinase from fission yeast.
Yang P, Kansra S, Pimental RA, Gilbreth M, Marcus S. Yang P, et al. J Biol Chem. 1998 Jul 17;273(29):18481-9. doi: 10.1074/jbc.273.29.18481. J Biol Chem. 1998. PMID: 9660817 - Controlling cell cycle progress in the fission yeast Schizosaccharomyces pombe.
MacNeill SA, Warbrick E, Fantes PA. MacNeill SA, et al. Curr Opin Genet Dev. 1991 Oct;1(3):307-12. doi: 10.1016/s0959-437x(05)80292-8. Curr Opin Genet Dev. 1991. PMID: 1840886 Review.
Cited by
- Functional Properties of the MAP Kinase UeKpp2 in Ustilago esculenta.
Zhang Y, Hu Y, Cao Q, Yin Y, Xia W, Cui H, Yu X, Ye Z. Zhang Y, et al. Front Microbiol. 2020 Jun 9;11:1053. doi: 10.3389/fmicb.2020.01053. eCollection 2020. Front Microbiol. 2020. PMID: 32582058 Free PMC article. - The tricornered gene, which is required for the integrity of epidermal cell extensions, encodes the Drosophila nuclear DBF2-related kinase.
Geng W, He B, Wang M, Adler PN. Geng W, et al. Genetics. 2000 Dec;156(4):1817-28. doi: 10.1093/genetics/156.4.1817. Genetics. 2000. PMID: 11102376 Free PMC article. - Gef1p, a new guanine nucleotide exchange factor for Cdc42p, regulates polarity in Schizosaccharomyces pombe.
Coll PM, Trillo Y, Ametzazurra A, Perez P. Coll PM, et al. Mol Biol Cell. 2003 Jan;14(1):313-23. doi: 10.1091/mbc.e02-07-0400. Mol Biol Cell. 2003. PMID: 12529446 Free PMC article. - Prevention of calpain-dependent degradation of STK38 by MEKK2-mediated phosphorylation.
Enomoto A, Fukasawa T, Tsumoto H, Karube M, Nakagawa K, Yoshizaki A, Sato S, Miura Y, Miyagawa K. Enomoto A, et al. Sci Rep. 2019 Nov 5;9(1):16010. doi: 10.1038/s41598-019-52435-8. Sci Rep. 2019. PMID: 31690749 Free PMC article. - Model of fission yeast cell shape driven by membrane-bound growth factors and the cytoskeleton.
Drake T, Vavylonis D. Drake T, et al. PLoS Comput Biol. 2013;9(10):e1003287. doi: 10.1371/journal.pcbi.1003287. Epub 2013 Oct 17. PLoS Comput Biol. 2013. PMID: 24146607 Free PMC article.
References
- Drubin D G, Nelson W J. Cell. 1996;84:335–344. - PubMed
- Mitchison J M, Nurse P. J Cell Sci. 1985;75:357–376. - PubMed
- Marks J, Hyams J S. Eur J Cell Biol. 1985;110:417–425.
- Nurse P. Nature (London) 1975;256:547–551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous