C/EBPbeta, but not C/EBPalpha, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland - PubMed (original) (raw)
C/EBPbeta, but not C/EBPalpha, is essential for ductal morphogenesis, lobuloalveolar proliferation, and functional differentiation in the mouse mammary gland
T N Seagroves et al. Genes Dev. 1998.
Abstract
The CCAAT/enhancer binding proteins (C/EBPs) are differentially expressed throughout mammary gland development and interact with binding sites within the promoter of a milk protein gene, beta-casein. The specific roles of C/EBPbeta and C/EBPalpha in mouse mammary gland development and differentiation have been investigated in mice that carry targeted deletions of these genes. C/EBPbeta-/- virgin mice exhibited cystic, enlarged mammary ducts with decreased secondary branching. Transplantation of C/EBPbeta-/- mammary epithelium into the cleared mammary fat pads of nude mice confirmed that this defect in ductal morphogenesis was intrinsic to the epithelium. When treated with estrogen/progesterone (E+P) to simulate pregnancy, C/EBPbeta-/- mammary glands displayed only limited lobuloalveolar development and ductal side branching. Primary mammary epithelial cells obtained from E+P-treated C/EBPbeta-/- mice that were cultured on extracellular matrix gels did not functionally differentiate in response to lactogenic hormones despite their organization into three-dimensional structures. Expression of beta-casein protein was inhibited 85%-100% and whey acidic protein (WAP) was undetectable. In contrast, no detectable alterations in mammary development or beta-casein expression were observed in mammary outgrowths derived from newborn C/EBPalpha-/- mammary epithelium transplanted into the cleared mammary fat pads of syngeneic hosts. These results demonstrate that C/EBPbeta, but not C/EBPalpha, is required for ductal morphogenesis, lobuloalveolar development, and functional differentiation of mammary epithelial cells.
Figures
Figure 1
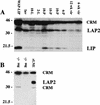
Expression of the C/EBPβ isoforms in C57/Bl6 mice during mammary development. (A) Mammary tissue was isolated from mice at the following developmental stages: immature virgin (6 week vir), mature virgin (12 week vir), 6 days of pregnancy (6-P), 10 days of pregnancy (10-P), 15 days of pregnancy (15-P), 18 days of pregnancy (18-P), 2 days of lactation (2-L), 10 days of lactation (10-L), and 10 days of lactation followed by 4 days of involution induced by removal of pups (Inv). Aliquots of WCE (100 μg) were separated by SDS-PAGE, transferred to membrane, and probed with antibodies against rat C/EBPβ (Santa Cruz, sc-150-x) as described in Materials and Methods. The LAP2 (36 kD) and LIP (20 kD) isoforms of C/EBPβ are shown (A,B). As a positive control for expression of LIP (A), an aliquot (100 μg) from a late pregnant WAP-driven LIP transgenic mouse (WAP–LIP) was included. (B) The 45-kD band referred to previously as LAP1 (Raught et al. 1995) is CRM as indicated by probing C/EBPβ−/− virgin mg (−/−mg) with C/EBPβ antibody. In addition, CRM at 30 kD was noted in some C/EBPβ−/− mammary gland extracts and in the rat liver nuclear extract (rLNE) included as a positive control (B).
Figure 2
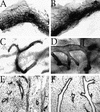
C/EBPβ−/− virgins exhibit abnormal, cystic ducts compared with wild-type littermates. Very large bloated ducts are obvious in whole mount preparations taken from mature C/EPBβ−/− mice (B,D) compared with glands isolated from wild-type littermates that contained normal ducts (A,C,E). Analysis of hematoxylin and eosin-stained paraffin-embedded sections (E,F) indicates that the enlarged ducts present in C/EBPβ nulls (F) are not the result of proteinaceous material trapped within the ducts or hyperplasia of the ductal epithelium. Images were captured at either 1.5× (A,B) or 10× (C_–_F) magnification.
Figure 3
Transplantation of epithelium from C/EBPβ donors of each genotype into the cleared fat pads of nu/nu recipients localizes the defect in ductal morphogenesis to the mammary epithelium. Six weeks post-transplantation, whole transplanted inguinal mammary glands were isolated from recipients, fixed and stained with hematoxylin for whole mount analysis. The C/EBPβ+/+ transplants (A,D) contain normal narrow branched ducts, whereas the C/EBPβ+/− (B,E) exhibit an intermediate phenotype with some bloated ducts, and the C/EPBβ−/− transplants (C,F) contain primarily the bloated ducts observed in intact C/EBPβ−/− virgins.
Figure 4
Lobuloalveolar development is impaired in the C/EBPβ−/− mice following E+P treatment. The thoracic glands from E+P-treated C/EBPβ+/− or C/EBPβ−/− mice were fixed and stained with hematoxylin by whole mount preparation. In contrast to extensive lobuloalveolar development observed in the C/EBPβ+/− glands (A), large areas of ductal epithelium in the C/EBPβ−/− glands (B) did not contain either secondary/tertiary side branches or alveoli.
Figure 5
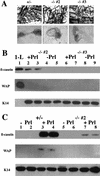
C/EBPβ−/− MECs isolated from E+P-treated females fail to functionally differentiate in response to lactogenic hormones (Prl, I, H). Primary mammary epithelial cells isolated from E+P-treated C/EBPβ+/− and two individual C/EPBβ−/− females (−/− #2 and −/− #3) were cultured on Matrigel (A) in duplicate sets of wells and treated with Prl+I+H as indicated in Materials and Methods. Portions of E+P-treated glands were whole mounted to confirm extent of lobuloalveolar development (A). Duplicate wells of cells isolated from each animal treated with I+H but without prolactin (−Prl) were included as noninduced controls (B, lanes 4,5,8,9; C, lanes 1,2,5,6). Both the heterozygous (+/−) and null (−/−) cells are capable of organizing into three-dimensional structures on Matrigel (A), however, the MECs from C/EBPβ−/− mice fail to produce WAP and expression of β-casein is inhibited 85%–100% (B, lanes 2,3,6,7; C, lanes 7,8). Expression of milk protein genes in the C/EBPβ−/− mice was compared with a heterozygous control (+/−, C, lanes 1_–_4) as described in Materials and Methods. Equivalent loading was determined by Western blotting with K14 antisera (B,C). No K14 was detected in the control from day 1 of lactation (B, 1-L) because of the high ratio of epithelial cells to myoepithelial cells at this stage of development and the low amounts of protein analyzed.
Figure 6
C/EBPα mRNA is expressed throughout development of the murine mammary gland. C/EBPα mRNA is detected during all stages of mammary development including in the mature virgin (vir), and during mid-pregnancy (13-P), late pregnancy (17-P), day 1 lactation (1-L), and mid-lactation (8-L). The expression of C/EBPα mRNA in the mammary gland is ∼20%–25% the level of C/EBPα detected in the mature rat liver (liver). When corrected for the increase of epithelial cells that occurs during development of the mammary gland, measured by the levels of K18 mRNA, the ratio of C/EBPα/K18 remains fairly constant during development. The ratio of C/EBPα/K18 at day 1 lactation (0.20) is not significantly different from that observed during mid-pregnancy (0.30) or late pregnancy (0.24). The apparent decrease in both C/EBPα and K18 that occurs at mid-lactation (8-L) is most likely a dilutional effect of the abundant milk protein mRNAs.
Figure 7
Mammary development is normal in the C/EBPα−/− transplants. Portions of mammary tissue isolated from wild-type (A,C,E,G) or C/EBPα null (B,D,F,H) transplanted glands were fixed and stained with Harris hematoxylin according to standard whole mount procedure. Glands from virgin (A,B), day 13 pregnant (C,D), day 1 lactation (E,F), and day 4 involuted mice (G,H) are included. The whole mounts from 17 days of pregnancy (data not shown) were omitted because they closely resembled the glands isolated at day 1 of lactation. Images of the glands were directly captured from a Sony video camera at 4× (A,B,E,F) or at 10× (C,D,G,H) magnification. Apparent differences in magnification are a result of the original size of each transplant gland. Note the presence of normal ducts (D) in the virgin outgrowths and the presence of terminal end buds in the portion of the ductal tree that has not yet reached the edge of the fat pad in the C/EBPα−/− gland taken at 6 weeks post-transplantation (B).
Figure 8
Histology of C/EBPα−/− transplants at day 1 of lactation is normal (A) and the C/EBPα−/− transplants make normal levels of β-casein (B). Paraffin-embedded sections from 1-day lactating animals stained with hematoxylin and eosin reveal that normal, secretory alveoli are present in both the C/EBPα−/− (−/−; 2) and wild-type transplants (+/+; 1). The C/EBPα+/+ (1_–_3) and C/EBPα−/− (4_–_6) transplants also express equivalent levels of β-casein as determined by Western blotting of 1 μg of WCE from these transplants (B).
Similar articles
- The C/EBPbeta transcription factor regulates epithelial cell proliferation and differentiation in the mammary gland.
Robinson GW, Johnson PF, Hennighausen L, Sterneck E. Robinson GW, et al. Genes Dev. 1998 Jun 15;12(12):1907-16. doi: 10.1101/gad.12.12.1907. Genes Dev. 1998. PMID: 9637691 Free PMC article. - Disruption of steroid and prolactin receptor patterning in the mammary gland correlates with a block in lobuloalveolar development.
Grimm SL, Seagroves TN, Kabotyanski EB, Hovey RC, Vonderhaar BK, Lydon JP, Miyoshi K, Hennighausen L, Ormandy CJ, Lee AV, Stull MA, Wood TL, Rosen JM. Grimm SL, et al. Mol Endocrinol. 2002 Dec;16(12):2675-91. doi: 10.1210/me.2002-0239. Mol Endocrinol. 2002. PMID: 12456789 - Expression and function of CCAAT/enhancer binding proteinbeta (C/EBPbeta) LAP and LIP isoforms in mouse mammary gland, tumors and cultured mammary epithelial cells.
Dearth LR, Hutt J, Sattler A, Gigliotti A, DeWille J. Dearth LR, et al. J Cell Biochem. 2001;82(3):357-70. doi: 10.1002/jcb.1167. J Cell Biochem. 2001. PMID: 11500913 - The role of C/EBPbeta in mammary gland development and breast cancer.
Grimm SL, Rosen JM. Grimm SL, et al. J Mammary Gland Biol Neoplasia. 2003 Apr;8(2):191-204. doi: 10.1023/a:1025900908026. J Mammary Gland Biol Neoplasia. 2003. PMID: 14635794 Review. - Delivering the message: epimorphin and mammary epithelial morphogenesis.
Radisky DC, Hirai Y, Bissell MJ. Radisky DC, et al. Trends Cell Biol. 2003 Aug;13(8):426-34. doi: 10.1016/s0962-8924(03)00146-6. Trends Cell Biol. 2003. PMID: 12888295 Free PMC article. Review.
Cited by
- IkappaB kinase alpha kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells.
Cao Y, Luo JL, Karin M. Cao Y, et al. Proc Natl Acad Sci U S A. 2007 Oct 2;104(40):15852-7. doi: 10.1073/pnas.0706728104. Epub 2007 Sep 21. Proc Natl Acad Sci U S A. 2007. PMID: 17890319 Free PMC article. - Gene expression profiling of mammary gland development reveals putative roles for death receptors and immune mediators in post-lactational regression.
Clarkson RW, Wayland MT, Lee J, Freeman T, Watson CJ. Clarkson RW, et al. Breast Cancer Res. 2004;6(2):R92-109. doi: 10.1186/bcr754. Epub 2003 Dec 18. Breast Cancer Res. 2004. PMID: 14979921 Free PMC article. - Peroxisome proliferator-activated receptor alpha activation during pregnancy severely impairs mammary lobuloalveolar development in mice.
Yang Q, Kurotani R, Yamada A, Kimura S, Gonzalez FJ. Yang Q, et al. Endocrinology. 2006 Oct;147(10):4772-80. doi: 10.1210/en.2006-0437. Epub 2006 Jul 20. Endocrinology. 2006. PMID: 16857745 Free PMC article. - Critical prosurvival roles for C/EBP beta and insulin-like growth factor I in macrophage tumor cells.
Wessells J, Yakar S, Johnson PF. Wessells J, et al. Mol Cell Biol. 2004 Apr;24(8):3238-50. doi: 10.1128/MCB.24.8.3238-3250.2004. Mol Cell Biol. 2004. PMID: 15060147 Free PMC article. - MicroRNA-191 targets CCAAT/enhanced binding protein β and functions as an oncogenic molecule in human non-small cell lung carcinoma cells.
Li F, Wen J, Shi J, Wang Y, Yang F, Liu C. Li F, et al. Exp Ther Med. 2019 Aug;18(2):1175-1183. doi: 10.3892/etm.2019.7668. Epub 2019 Jun 13. Exp Ther Med. 2019. PMID: 31316611 Free PMC article.
References
- Alam T, An MR, Papaconstantinou J. Differential expression of three C/EBP isoforms in multiple tissues during the acute phase response. J Biol Chem. 1992;267:5021–5024. - PubMed
- Bissell MJ, Aggeler J. Dynamic reciprocity: How do extracellular matrix and hormones direct gene expression? Prog Clin Biol Res. 1987;249:251–262. - PubMed
- Blais S, Boudreau F, Beaulieu JF, Asselin C. CCAAT/enhancer binding protein isoforms expression in the colon of neonatal mice. Dev Dyn. 1995;204:66–76. - PubMed
- Cao Z, Umek RM, McKnight SL. Regulated expression of three C/EBP isoforms during adipose conversion of 3T3-L1 cells. Genes & Dev. 1991;5:1538–1552. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases