Identification of functional exonic splicing enhancer motifs recognized by individual SR proteins - PubMed (original) (raw)
Identification of functional exonic splicing enhancer motifs recognized by individual SR proteins
H X Liu et al. Genes Dev. 1998.
Abstract
Using an in vitro randomization and functional selection procedure, we have identified three novel classes of exonic splicing enhancers (ESEs) recognized by human SF2/ASF, SRp40, and SRp55, respectively. These ESEs are functional in splicing and are highly specific. For SF2/ASF and SRp55, in most cases, only the cognate SR protein can efficiently recognize an ESE and activate splicing. In contrast, the SRp40-selected ESEs can function with either SRp40 or SRp55, but not with SF2/ASF. UV cross-linking/competition and immunoprecipitation experiments showed that SR proteins recognize their cognate ESEs in nuclear extract by direct and specific binding. A motif search algorithm was used to derive consensus sequences for ESEs recognized by these SR proteins. Each SR protein yielded a distinct 5- to 7-nucleotide degenerate consensus. These three consensus sequences occur at higher frequencies in exons than in introns and may thus help define exon-intron boundaries. They occur in clusters within regions corresponding to naturally occurring, mapped ESEs. We conclude that a remarkably diverse set of sequences can function as ESEs. The degeneracy of these motifs is consistent with the fact that exonic enhancers evolved within extremely diverse protein coding sequences and are recognized by a small number of SR proteins that bind RNA with limited sequence specificity.
Figures
Figure 1
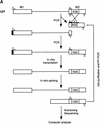
Procedure for randomization and selection of ESEs. (A) The natural ESE in mouse IgM exon M2 was replaced by a 20-nucleotide segment of random sequence, and a library of pre-mRNAs was constructed by overlap–extension PCR and in vitro transcription. A sample of this pool, representing ∼1.2 × 1010 pre-mRNA molecules was then spliced in vitro by complementation of an S100 extract with individual recombinant SR proteins. The pool of spliced mRNA products was gel purified, and the sequences corresponding to the ESE region were rebuilt into pre-mRNA template molecules for a new round of selection, or subcloned and sequenced. The sequences were analyzed by a motif-search algorithm to identify common patterns. (B) Sequence of the M2 exon of the mouse IgM gene. The sequence of the previously mapped ESE is shown in uppercase.
Figure 1
Procedure for randomization and selection of ESEs. (A) The natural ESE in mouse IgM exon M2 was replaced by a 20-nucleotide segment of random sequence, and a library of pre-mRNAs was constructed by overlap–extension PCR and in vitro transcription. A sample of this pool, representing ∼1.2 × 1010 pre-mRNA molecules was then spliced in vitro by complementation of an S100 extract with individual recombinant SR proteins. The pool of spliced mRNA products was gel purified, and the sequences corresponding to the ESE region were rebuilt into pre-mRNA template molecules for a new round of selection, or subcloned and sequenced. The sequences were analyzed by a motif-search algorithm to identify common patterns. (B) Sequence of the M2 exon of the mouse IgM gene. The sequence of the previously mapped ESE is shown in uppercase.
Figure 2
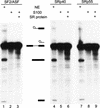
Splicing of the pre-mRNA pool prior to selection. Radiolabeled pre-mRNA (20 fmoles) from the unselected initial pool was incubated under splicing conditions in nuclear extract (lanes 1,4,7), in S100 extract only (lanes 2,5,8), or in S100 extract complemented with the indicated SR protein (lanes 3,6,9). The RNAs were analyzed by denaturing PAGE (12% polyacrylamide) and autoradiography. The structures and mobilities of the precursor, intermediates, and products of splicing (Watakabe et al. 1993) are shown next to each autoradiograph.
Figure 3
Sequence alignment of SF2/ASF-specific ESEs (A) and sequences from the initial random pool (B). A consensus motif was identified as described in the text. The sequences are aligned on the basis of the best fit to the consensus within each sequence. Nucleotides in the boxed alignment that match the consensus position are shown white on black; mismatched nucleotides are not shaded. Underlined nucleotides are from the constant region flanking the randomized segment. The nucleotide composition of the randomized segment in the selected pool is provided in the lower left corner. S = G or C; R = A or G. Clones indicated in parentheses were selected for further characterization.
Figure 3
Sequence alignment of SF2/ASF-specific ESEs (A) and sequences from the initial random pool (B). A consensus motif was identified as described in the text. The sequences are aligned on the basis of the best fit to the consensus within each sequence. Nucleotides in the boxed alignment that match the consensus position are shown white on black; mismatched nucleotides are not shaded. Underlined nucleotides are from the constant region flanking the randomized segment. The nucleotide composition of the randomized segment in the selected pool is provided in the lower left corner. S = G or C; R = A or G. Clones indicated in parentheses were selected for further characterization.
Figure 4
Sequence alignment of SRp40-specific ESEs. D = A, G, or U. For details, see Fig. 3 legend.
Figure 5
Sequence alignment of SRp55-specific ESEs. K = U or G; M = A or C. For details, see Fig. 3 legend.
Figure 6
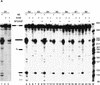
The selected sequences are functional SR protein-dependent ESEs. (A) In vitro splicing of pre-mRNAs containing the SF2/ASF-selected winner sequences in HeLa nuclear extract (lanes 1,4,7,10,13,16,19,22), S100 extract alone (lanes 2,5,8,11,14, 17,20,23), or S100 complemented by 20 pmoles of recombinant SF2/ASF (lanes 3,6,9,12,15,18,21,24). (B) Splicing of SRp40-selected winner sequences in nuclear extract (lanes 1,4,7,10, 13,16,19), S100 extract alone (lanes 2,5,8,11,14,17,20), or S100 extract complemented by 20 pmoles of recombinant SRp40 (lanes 3,6,9,12,15,18,21). (C) Splicing of SRp55-selected winner sequences in nuclear extract (lanes 1,5,9,13,17,21,25,29), S100 extract alone (lanes 2,6,10,14,18,22,26,30), or in S100 extract complemented by 10 pmoles SRp55 (lanes 3,7,11,15,19,23,27,31) or by 20 pmoles of SRp55 (lanes 4,8,12,16,20,24,28,32). The RNAs were analyzed by denaturing PAGE (5.5% polyacrylamide) and autoradiography. The structures and mobilities of the precursor, intermediates, and products of splicing (Watakabe et al. 1993) are shown next to each autoradiograph.
Figure 6
The selected sequences are functional SR protein-dependent ESEs. (A) In vitro splicing of pre-mRNAs containing the SF2/ASF-selected winner sequences in HeLa nuclear extract (lanes 1,4,7,10,13,16,19,22), S100 extract alone (lanes 2,5,8,11,14, 17,20,23), or S100 complemented by 20 pmoles of recombinant SF2/ASF (lanes 3,6,9,12,15,18,21,24). (B) Splicing of SRp40-selected winner sequences in nuclear extract (lanes 1,4,7,10, 13,16,19), S100 extract alone (lanes 2,5,8,11,14,17,20), or S100 extract complemented by 20 pmoles of recombinant SRp40 (lanes 3,6,9,12,15,18,21). (C) Splicing of SRp55-selected winner sequences in nuclear extract (lanes 1,5,9,13,17,21,25,29), S100 extract alone (lanes 2,6,10,14,18,22,26,30), or in S100 extract complemented by 10 pmoles SRp55 (lanes 3,7,11,15,19,23,27,31) or by 20 pmoles of SRp55 (lanes 4,8,12,16,20,24,28,32). The RNAs were analyzed by denaturing PAGE (5.5% polyacrylamide) and autoradiography. The structures and mobilities of the precursor, intermediates, and products of splicing (Watakabe et al. 1993) are shown next to each autoradiograph.
Figure 6
The selected sequences are functional SR protein-dependent ESEs. (A) In vitro splicing of pre-mRNAs containing the SF2/ASF-selected winner sequences in HeLa nuclear extract (lanes 1,4,7,10,13,16,19,22), S100 extract alone (lanes 2,5,8,11,14, 17,20,23), or S100 complemented by 20 pmoles of recombinant SF2/ASF (lanes 3,6,9,12,15,18,21,24). (B) Splicing of SRp40-selected winner sequences in nuclear extract (lanes 1,4,7,10, 13,16,19), S100 extract alone (lanes 2,5,8,11,14,17,20), or S100 extract complemented by 20 pmoles of recombinant SRp40 (lanes 3,6,9,12,15,18,21). (C) Splicing of SRp55-selected winner sequences in nuclear extract (lanes 1,5,9,13,17,21,25,29), S100 extract alone (lanes 2,6,10,14,18,22,26,30), or in S100 extract complemented by 10 pmoles SRp55 (lanes 3,7,11,15,19,23,27,31) or by 20 pmoles of SRp55 (lanes 4,8,12,16,20,24,28,32). The RNAs were analyzed by denaturing PAGE (5.5% polyacrylamide) and autoradiography. The structures and mobilities of the precursor, intermediates, and products of splicing (Watakabe et al. 1993) are shown next to each autoradiograph.
Figure 7
SR protein specificity of in vitro-selected ESEs. (A) SRp40-selected ESEs are inactive with SF2/ASF. Splicing was carried out in HeLa S100 extract complemented with 20 pmoles of SF2/ASF (lanes 1,4,7,10,13,16,19), 20 pmoles of SRp40 (lanes 2,5,8,11,14,17,20), or 20 pmoles of SF2/ASF plus 20 pmoles of SRp40 (lanes 3,6,9,12,15,18,21). (B) SRp40-selected ESEs can function in the presence of SRp55. Splicing was carried out in S100 extract complemented with 20 pmoles of SRp55 (lanes 1,4,7,10,13,16), 20 pmoles of SRp40 (lanes 2,5,8,11,14,17), or 20 pmoles of SRp55 plus 20 pmoles of SRp40 (lanes 3,6,9,12,15,18).
Figure 7
SR protein specificity of in vitro-selected ESEs. (A) SRp40-selected ESEs are inactive with SF2/ASF. Splicing was carried out in HeLa S100 extract complemented with 20 pmoles of SF2/ASF (lanes 1,4,7,10,13,16,19), 20 pmoles of SRp40 (lanes 2,5,8,11,14,17,20), or 20 pmoles of SF2/ASF plus 20 pmoles of SRp40 (lanes 3,6,9,12,15,18,21). (B) SRp40-selected ESEs can function in the presence of SRp55. Splicing was carried out in S100 extract complemented with 20 pmoles of SRp55 (lanes 1,4,7,10,13,16), 20 pmoles of SRp40 (lanes 2,5,8,11,14,17), or 20 pmoles of SRp55 plus 20 pmoles of SRp40 (lanes 3,6,9,12,15,18).
Figure 8
Specific binding of SF2/ASF to an SF2/ASF-selected ESE. (A) UV cross-linking competition binding assay. Radiolabeled exon M2 RNA (20 fmoles) comprising the B1 winner sequence (B1E) was incubated under splicing conditions in HeLa nuclear extract. Subsequent UV cross-linking and RNase digestion resulted in label transfer predominantly to two proteins of 34 and 50 kD. The former, which binds specifically, is presumed to be SF2/ASF (see below). Cold competitor RNAs containing either the B1 winner insert, an SRp55-selected insert (C4E), an SRp40-selected insert (E7E), or other control sequence inserts, were present in excess, as indicated above the autoradiograph. (Lane 1) No competitor; in the remaining lanes, the indicated competitors were present in 5-fold excess (even lanes) or 50-fold excess (odd lanes) over the labeled B1E RNA. (B) Immunoprecipitation of SF2/ASF UV cross-linked to the B1E RNA. UV cross-linking was carried out as in A, lane 1. A 5% equivalent of the input was loaded directly (lane 1). Parallel reactions were incubated with a control antibody (lane 3), or with anti-SF2/ASF monoclonal antibody (lane 2), and the immunoprecipitates were recovered in SDS gel loading buffer. In A and B, the samples were analyzed by SDS-PAGE and autoradiography.
Figure 8
Specific binding of SF2/ASF to an SF2/ASF-selected ESE. (A) UV cross-linking competition binding assay. Radiolabeled exon M2 RNA (20 fmoles) comprising the B1 winner sequence (B1E) was incubated under splicing conditions in HeLa nuclear extract. Subsequent UV cross-linking and RNase digestion resulted in label transfer predominantly to two proteins of 34 and 50 kD. The former, which binds specifically, is presumed to be SF2/ASF (see below). Cold competitor RNAs containing either the B1 winner insert, an SRp55-selected insert (C4E), an SRp40-selected insert (E7E), or other control sequence inserts, were present in excess, as indicated above the autoradiograph. (Lane 1) No competitor; in the remaining lanes, the indicated competitors were present in 5-fold excess (even lanes) or 50-fold excess (odd lanes) over the labeled B1E RNA. (B) Immunoprecipitation of SF2/ASF UV cross-linked to the B1E RNA. UV cross-linking was carried out as in A, lane 1. A 5% equivalent of the input was loaded directly (lane 1). Parallel reactions were incubated with a control antibody (lane 3), or with anti-SF2/ASF monoclonal antibody (lane 2), and the immunoprecipitates were recovered in SDS gel loading buffer. In A and B, the samples were analyzed by SDS-PAGE and autoradiography.
Figure 9
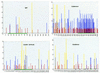
Distribution of in vitro-selected ESE consensus sequences within exons comprising natural ESEs. Score matrices were built for each class of in vitro-selected ESE, as described in Materials and Methods. The indicated natural exon sequences were searched with each score matrix, and the resulting scores (_y_-axis) were plotted against the nucleotide positions for each exon (x axis). Note that the _x_-axis scales are different in each case, because of the different exon sizes. Graphs are shown for mouse IgM exon M2, bovine growth hormone 3′ exon, Drosophila dsx female-specific exon, and chicken caldesmon exon 5. High score motif matches are shown by blue (SF2/ASF), red (SRp40), and yellow (SRp55) vertical bars. For each SR protein, only the sequence matches with a score greater than that of the lowest scoring winner sequence in Figs. 3–5 are shown. Note that there is no relation between the height of bars of different colors. The green horizontal bars under the x axis indicate previously mapped ESEs or the dsx PRE. The black horizontal bars denote the dsx repeat elements (dsxREs).
Similar articles
- Distribution of SR protein exonic splicing enhancer motifs in human protein-coding genes.
Wang J, Smith PJ, Krainer AR, Zhang MQ. Wang J, et al. Nucleic Acids Res. 2005 Sep 7;33(16):5053-62. doi: 10.1093/nar/gki810. Print 2005. Nucleic Acids Res. 2005. PMID: 16147989 Free PMC article. - Exonic splicing enhancer motif recognized by human SC35 under splicing conditions.
Liu HX, Chew SL, Cartegni L, Zhang MQ, Krainer AR. Liu HX, et al. Mol Cell Biol. 2000 Feb;20(3):1063-71. doi: 10.1128/MCB.20.3.1063-1071.2000. Mol Cell Biol. 2000. PMID: 10629063 Free PMC article. - ESEfinder: A web resource to identify exonic splicing enhancers.
Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. Cartegni L, et al. Nucleic Acids Res. 2003 Jul 1;31(13):3568-71. doi: 10.1093/nar/gkg616. Nucleic Acids Res. 2003. PMID: 12824367 Free PMC article. - Evidence for the function of an exonic splicing enhancer after the first catalytic step of pre-mRNA splicing.
Chew SL, Liu HX, Mayeda A, Krainer AR. Chew SL, et al. Proc Natl Acad Sci U S A. 1999 Sep 14;96(19):10655-60. doi: 10.1073/pnas.96.19.10655. Proc Natl Acad Sci U S A. 1999. PMID: 10485881 Free PMC article. - Exonic splicing enhancers: mechanism of action, diversity and role in human genetic diseases.
Blencowe BJ. Blencowe BJ. Trends Biochem Sci. 2000 Mar;25(3):106-10. doi: 10.1016/s0968-0004(00)01549-8. Trends Biochem Sci. 2000. PMID: 10694877 Review.
Cited by
- A Quantitative Profiling Tool for Diverse Genomic Data Types Reveals Potential Associations between Chromatin and Pre-mRNA Processing.
Kremsky I, Bellora N, Eyras E. Kremsky I, et al. PLoS One. 2015 Jul 24;10(7):e0132448. doi: 10.1371/journal.pone.0132448. eCollection 2015. PLoS One. 2015. PMID: 26207626 Free PMC article. - Distribution of SR protein exonic splicing enhancer motifs in human protein-coding genes.
Wang J, Smith PJ, Krainer AR, Zhang MQ. Wang J, et al. Nucleic Acids Res. 2005 Sep 7;33(16):5053-62. doi: 10.1093/nar/gki810. Print 2005. Nucleic Acids Res. 2005. PMID: 16147989 Free PMC article. - Pre-mRNA splicing by the essential Drosophila protein B52: tissue and target specificity.
Hoffman BE, Lis JT. Hoffman BE, et al. Mol Cell Biol. 2000 Jan;20(1):181-6. doi: 10.1128/MCB.20.1.181-186.2000. Mol Cell Biol. 2000. PMID: 10594020 Free PMC article. - Serine/arginine-rich proteins contribute to negative regulator of splicing element-stimulated polyadenylation in rous sarcoma virus.
Maciolek NL, McNally MT. Maciolek NL, et al. J Virol. 2007 Oct;81(20):11208-17. doi: 10.1128/JVI.00919-07. Epub 2007 Aug 1. J Virol. 2007. PMID: 17670832 Free PMC article. - Exonic splicing enhancer motif recognized by human SC35 under splicing conditions.
Liu HX, Chew SL, Cartegni L, Zhang MQ, Krainer AR. Liu HX, et al. Mol Cell Biol. 2000 Feb;20(3):1063-71. doi: 10.1128/MCB.20.3.1063-1071.2000. Mol Cell Biol. 2000. PMID: 10629063 Free PMC article.
References
- Burset M, Guigo R. Evaluation of gene structure prediction programs. Genomics. 1996;34:353–367. - PubMed
- Cáceres JF, Krainer AR. Mammalian pre-mRNA splicing factors. In: Krainer AR, editor. Eukaryotic mRNA processing. Oxford, UK: IRL Press; 1997. pp. 174–212.
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM042699/GM/NIGMS NIH HHS/United States
- R37 GM042699/GM/NIGMS NIH HHS/United States
- Z01 HG000010/ImNIH/Intramural NIH HHS/United States
- GM42699/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials