Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury - PubMed (original) (raw)
Lasting N-terminal phosphorylation of c-Jun and activation of c-Jun N-terminal kinases after neuronal injury
T Herdegen et al. J Neurosci. 1998.
Abstract
Transcription factor c-Jun is proposed to control neuronal cell death and survival, but its activation by N-terminal phosphorylation and the underlying activity of the c-Jun N-terminal kinases (JNKs) remain to be elucidated in the adult mammalian brain. We generated a polyclonal antiserum that specifically recognizes c-Jun phosphorylated at its serine 73 (S73) residue after UV irradiation of 3T3 cells. Disruption of the c-jun locus in 3T3 cells abolished this reaction, and retransfection of the human c-jun at the c-jun-/- background restored it. The phospho-c-Jun antiserum was used to visualize N-terminally phosphorylated c-Jun in the adult rat brain with cellular resolution. Prolonged c-Jun S73 phosphorylation was detected in affected neurons up to 5 d after transient occlusion of medial cerebral artery or up to 50 d after transection of central nerve fiber tracts. After cerebral ischemia-reperfusion, phosphorylation of c-Jun was linked with induced expression of Fas-ligand (APO-1, CD95-ligand), whose gene is a putative c-Jun/AP-1 target, and with terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end labeling (TUNEL) reactivity, a marker for apoptosis. After nerve fiber transection, however, lasting c-Jun phosphorylation occurred in axotomized neurons negative for Fas-ligand or TUNEL and regardless of degeneration or survival. In contrast to these lasting phosphorylation patterns, transient seizure activity by pentylenetetrazole provoked only a brief c-Jun phosphorylation and JNK activation. In extracts from ischemic or axotomized brain compartments, c-Jun phosphorylation correlated with enhanced long-term JNK activity, and in-gel kinase assays visualized proteins with sizes corresponding to JNK isoforms as the only c-Jun N-terminally phosphorylating enzymes. These results demonstrate that lasting c-Jun S73 phosphorylation and JNK activity are part of neuronal stress response after neurodegenerative disorders in the adult mammalian brain with Fas-ligand as a putative apoptotic effector.
Figures
Fig. 1.
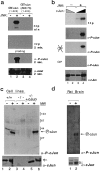
Characterization of the anti-phospho-c-Jun antiserum. a, GST-c-Jun(1–223) (lanes 1, 2) or GST-c-Jun(1–223, A63/73) (lanes 3, 4) were (+) or were not (−) phosphorylated by recombinant JNK2 in the presence of [32P]ATP.(Top to bottom) first panel, Autoradiogram of32P-labeled proteins exposed either overnight (o/n) or (second panel) exposed only for 2 min. Third panel, The same blot was probed with affinity-purified phospho-c-Jun (α_-P-cJun_) antibody. Fourth panel, The blot was stripped and reprobed with the c-Jun antiserum (α_-cJun_).b, Samples (0.1 μg, lanes 2, 3; 1.0 μg, lanes 1, 4) of recombinant full-length c-Jun (Deng and Karin, 1992) were not (lanes 1, 2) or were phosphorylated (lanes 3, 4) with recombinant JNK2 in the presence of [32P]ATP (lanes 3, 4). (Top to bottom) first panel, Autoradiogram of the 32P-labeled proteins. Second panel, Immunoblotting with the phospho-c-Jun antiserum (α_-P-cJun_). Third panel, The blot was stripped and treated with buffer containing heat-inactivated calf intestinal phosphatase ( ) or (fourth panel) native CIP (40 U/ml).Fifth panel, After final stripping, the blot was reprobed with the c-Jun antiserum (α_-cJun_).c, Immunodetection of phosphorylated c-Jun in immortalized 3T3 fibroblasts derived from wild-type (lanes 1, 2) or c-jun−/− mouse embryos (lanes 3, 4) (Hilberg et al., 1993) or c-jun−/−_cells stably transfected with a human c-jun expression vector (lanes 5, 6), which were (+) or were not (−) UV-irradiated. The membrane was probed with the phospho-c-Jun antiserum (α_-P-cJun) or c-Jun antibody (α_-cJun_). d, Detection of phosphorylated c-Jun in nuclear cortical extracts from untreated rats (lane 1) or after ischemia with 24 hr reperfusion (lane 2) by immunoblotting with the anti-phospho-c-Jun or anti-c-Jun antiserum.
) or (fourth panel) native CIP (40 U/ml).Fifth panel, After final stripping, the blot was reprobed with the c-Jun antiserum (α_-cJun_).c, Immunodetection of phosphorylated c-Jun in immortalized 3T3 fibroblasts derived from wild-type (lanes 1, 2) or c-jun−/− mouse embryos (lanes 3, 4) (Hilberg et al., 1993) or c-jun−/−_cells stably transfected with a human c-jun expression vector (lanes 5, 6), which were (+) or were not (−) UV-irradiated. The membrane was probed with the phospho-c-Jun antiserum (α_-P-cJun) or c-Jun antibody (α_-cJun_). d, Detection of phosphorylated c-Jun in nuclear cortical extracts from untreated rats (lane 1) or after ischemia with 24 hr reperfusion (lane 2) by immunoblotting with the anti-phospho-c-Jun or anti-c-Jun antiserum.
Fig. 2.
Immunodetection of c-Jun N-terminal phosphorylation in the adult rat brain. c-Jun-IR (a–d) and phospho-c-Jun-IR (e–h) in the mamillary nucleus (mm) are shown: a, e, untreated rats;b, f, 5 d after transection of the mamillothalamic tract; c, g, competition by preincubation of the antibodies with 100 pmol of the phosphorylated c-Jun peptide; d, h, preincubation of the section with 1.2 μU alkaline phosphatase before incubation with the antiserum; longitudinal (i) and (j) coronal aspect of the location site of the medial forebrain bundle (MFB) and mamillothalamic tract (MT) transection at bregma −2.3 and 1.5 mm lateral from midline. Scale bar, 200 μm.
Fig. 3.
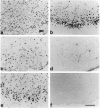
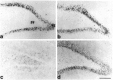
Phospho-c-Jun-IR in the SNC. a–c, c-Jun-IR and (d–f) phospho-c-Jun-IR in the SNC of (a, d) untreated animals, (b, e) 5 d or (c, f) 20 d after transection of the medial forebrain bundle. The dotted line separates the pars compacta (p.c.) and the pars reticularis (p.r.). Arrows mark labeled nuclei. Scale bar, 100 μm.
Fig. 4.
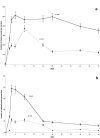
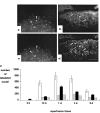
Time course of c-Jun and phosphorylated c-Jun in SNC and MnM after axotomy. Mean (±SD) of nuclei (per 50 μm section) labeled by (a) c-Jun and (b) phospho-c-Jun in the SNC (dotted line) and MnM (solid line) after transection of the medial forebrain bundle and mamillothalamic tract, respectively.
Fig. 5.
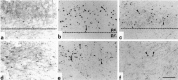
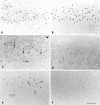
Expression and phosphorylation of c-Jun, and TUNEL staining after MCA occlusion. Shown are (a, b) c-Jun-IR, (c, d) phospho-c-Jun-IR, and (e, f) TUNEL reaction in the ipsilateral (a, c, e) and contralateral (b, d, f) piriform cortex of consecutive sections after MCA occlusion with reperfusion for 3 d. Scale bar, 75 μm.
Fig. 6.
Co-labeling of c-Jun phosphorylation and TUNEL. Shown is double-immunofluorescence of (a, b) phospho-c-Jun and (c, d) TUNEL in the superficial layer of the ipsilateral piriform cortex 12 hr (a, c) and 3 d (b, d) after MCA occlusion.Arrows indicate some of the double-labeled nuclei.e, Numbers of neurons labeled by TUNEL (white bars) and phospho-c-Jun (black bars) in the piriform cortex ipsilateral to the site of ischemia (between bregma −1.30 and −2.30). The time course gives the reperfusion period after MCA occlusion, which lasted 90 min. The numbers_represent mean (±SD) calculated from nine 35-μm-thick sections (three sections each of three rat brains per time point). The_gray bars give the proportion of TUNEL or phospho-c-Jun-labeled neurons that are co-labeled with phospho-c-Jun or TUNEL, respectively.
Fig. 7.
c-Jun expression and phosphorylation after pentylenetetrazole-induced seizures. a, b, Expression of c-Jun and (c, d) phosphorylation of c-Jun in the dentate gyrus (dg) of (a, c) untreated rats and (b, d) 15 min after injection of PTZ. py, Pyramidal layer. Scale bar, 200 μm.
Fig. 8.
Expression of Fas-ligand in the penumbra after ischemia. Fas-ligand immunoreactivity in the piriform cortex (a) adjacent to the necrotic area that is marked by the dotted line and (b) in the contralateral intact cortex. Scale bar, 200 μm.
Fig. 9.
Phospho-c-Jun, Fas-ligand, and TUNEL in the SNC. Shown are (a, b) phospho-c-Jun immunoreactivity, (c, d) Fas-ligand immunoreactivity, and (e, f) TUNEL staining in the ipsilateral SNC 3 d after (a, c, e) MCA occlusion or (b, d, f) 10 d after transection of the MFB. Scale bar, 100 μm.
Fig. 10.
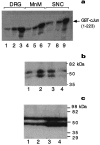
Activation of JNK. a, JNK-1 assay with GST-c-Jun (1–223) as substrate from dorsal root ganglia (DRG) extracts after sciatic nerve cut (lanes 1–3), in MnM after MT transection (lanes 4–6), and in SNC after MFB transection (lanes 7–9). Tissues were isolated from untreated controls (lanes 1, 4, 7), 3 d (lanes 2, 5, 8), or 12 d (lanes 3, 6, 9) after axotomy.b, In-gel kinase assays using GST-c-Jun (1–79) as substrate were performed with nuclear extracts from hippocampus and cortex isolated from untreated rats (lane 1) or 5 min (lane 2), 10 min (lane 3), and 90 min (lane 4) after intraperitoneal injection of pentylenetetrazole. c, In-gel kinase assay using GST-c-Jun (1–79) as substrate from hippocampus or piriform cortex microdissected from untreated rats (lanes 1, 2) or 24 hr after ischemia–reperfusion (lanes 3, 4). The autoradiograms in b and c did not contain additional bands.
Similar articles
- The mitochondrial toxin 3-nitropropionic acid induces striatal neurodegeneration via a c-Jun N-terminal kinase/c-Jun module.
Garcia M, Vanhoutte P, Pages C, Besson MJ, Brouillet E, Caboche J. Garcia M, et al. J Neurosci. 2002 Mar 15;22(6):2174-84. doi: 10.1523/JNEUROSCI.22-06-02174.2002. J Neurosci. 2002. PMID: 11896157 Free PMC article. - The c-Jun N-terminal protein kinase signaling pathway mediates Bax activation and subsequent neuronal apoptosis through interaction with Bim after transient focal cerebral ischemia.
Okuno S, Saito A, Hayashi T, Chan PH. Okuno S, et al. J Neurosci. 2004 Sep 8;24(36):7879-87. doi: 10.1523/JNEUROSCI.1745-04.2004. J Neurosci. 2004. PMID: 15356200 Free PMC article. - Activation of the c-Jun transcription factor following neurodegeneration in vivo.
Schenkel J. Schenkel J. Neurosci Lett. 2004 May 6;361(1-3):36-9. doi: 10.1016/j.neulet.2003.12.011. Neurosci Lett. 2004. PMID: 15135887 Review. - From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance.
Karin M, Gallagher E. Karin M, et al. IUBMB Life. 2005 Apr-May;57(4-5):283-95. doi: 10.1080/15216540500097111. IUBMB Life. 2005. PMID: 16036612 Review.
Cited by
- c-Jun-dependent CD95-L expression is a rate-limiting step in the induction of apoptosis by alkylating agents.
Kolbus A, Herr I, Schreiber M, Debatin KM, Wagner EF, Angel P. Kolbus A, et al. Mol Cell Biol. 2000 Jan;20(2):575-82. doi: 10.1128/MCB.20.2.575-582.2000. Mol Cell Biol. 2000. PMID: 10611236 Free PMC article. - A critical role of neural-specific JNK3 for ischemic apoptosis.
Kuan CY, Whitmarsh AJ, Yang DD, Liao G, Schloemer AJ, Dong C, Bao J, Banasiak KJ, Haddad GG, Flavell RA, Davis RJ, Rakic P. Kuan CY, et al. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):15184-9. doi: 10.1073/pnas.2336254100. Epub 2003 Dec 1. Proc Natl Acad Sci U S A. 2003. PMID: 14657393 Free PMC article. - Activation of mitogen-activated protein kinases after transient forebrain ischemia in gerbil hippocampus.
Sugino T, Nozaki K, Takagi Y, Hattori I, Hashimoto N, Moriguchi T, Nishida E. Sugino T, et al. J Neurosci. 2000 Jun 15;20(12):4506-14. doi: 10.1523/JNEUROSCI.20-12-04506.2000. J Neurosci. 2000. PMID: 10844020 Free PMC article. - Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70.
Giffard RG, Han RQ, Emery JF, Duan M, Pittet JF. Giffard RG, et al. Anesthesiology. 2008 Aug;109(2):339-48. doi: 10.1097/ALN.0b013e31817f4ce0. Anesthesiology. 2008. PMID: 18648242 Free PMC article. Review. - Increased operant responding for ethanol in male C57BL/6J mice: specific regulation by the ERK1/2, but not JNK, MAP kinase pathway.
Faccidomo S, Besheer J, Stanford PC, Hodge CW. Faccidomo S, et al. Psychopharmacology (Berl). 2009 May;204(1):135-47. doi: 10.1007/s00213-008-1444-9. Epub 2009 Jan 6. Psychopharmacology (Berl). 2009. PMID: 19125235 Free PMC article.
References
- Anderson AJ, Cummings BJ, Cotman CW. Increased immunoreactivity for Jun- and Fos-related proteins in Alzheimer’s disease: association with pathology. Exp Neurol. 1994;125:286–295. - PubMed
- Arias J, Alberts AS, Brindle P, Claret FX, Smeal T, Karin M, Feramisco J, Montminy M. Activation of cAMP and mitogen responsive genes relies on a common nuclear factor. Nature. 1994;370:226–229. - PubMed
- Asanuma M, Nishibayashi S, Kondo Y, Iwata E, Tsuda M, Ogawa N. Effects of single cyclosporin A pretreatment on pentylenetetrazol-induced convulsion and on TRE-binding activity in the rat brain. Mol Brain Res. 1995;33:29–36. - PubMed
- Blottner D, Herdegen T. Neuroprotective FGF-2 down-regulates the c-Jun transcription factor in axotomized sympathetic preganglionic neurons of adult rats. Neuroscience. 1997;82:283–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA 54418/CA/NCI NIH HHS/United States
- HL 35018/HL/NHLBI NIH HHS/United States
- R01 ES006376/ES/NIEHS NIH HHS/United States
- P01 CA054418/CA/NCI NIH HHS/United States
- ES06376/ES/NIEHS NIH HHS/United States
- P01 HL035018/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous