Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation - PubMed (original) (raw)
Identification of the dopamine D3 receptor in oligodendrocyte precursors: potential role in regulating differentiation and myelin formation
E R Bongarzone et al. J Neurosci. 1998.
Abstract
Expression of the dopamine D3 receptor (D3r) was found in primary mixed glial cultures from newborn brain and in the corpus callosum in vivo during the peak of myelination. Expression of the D3r mRNA, but not D2r mRNA, was detected as early as 5 d in vitro (DIV) by RT-PCR. Immunoblot studies revealed D3r protein was also expressed in the cultures. Double immunofluorescence analysis for the D3r and for surface markers of specific stages of oligodendrocyte development indicated that D3r expression occurred in precursors and in immature oligodendrocytes but not in mature oligodendrocytes (i.e. , A2B5(+) 007(-) 01(-) and A2B5(+) 007(+) 01(-) cells but not A2B5(-) 007(+) 01(+) cells). Confocal microscopic analysis indicated that D3r was associated with cell bodies and cell membranes but not with the processes emanating from cell somas. Immunohistochemistry of brain sections revealed the presence of D3r in some oligodendrocytes located mainly within the genu and radiato of the corpus callosum during the active period of myelination. Treatment of cultures with 20 microM quinpirole led to decreased numbers of O1(+) oligodendrocytes possessing myelin-like membranes as well as an increase in the number of precursors in 14 DIV cultures. This effect was prevented by the dopamine antagonist haloperidol. These results show that the D3r expression is not restricted to neurons but it is also expressed in differentiating oligodendrocytes before terminal maturation. It also suggests that dopamine or some other D3r ligand may play a role in oligodendrocyte differentiation and/or the formation of myelin by mature oligodendrocytes.
Figures
Fig. 1.
Primary cultures of glia express the D3r but not the D2r mRNA. The message for both dopamine receptors was detected by RT-PCR analysis. A, The scheme illustrates the structural organization of the D2r and D3r genes and their mRNAs. Two sets of specific oligonucleotide primers (open arrows, sense primers; filled arrows, antisense primers) were designed to amplify the cDNAs containing the open reading frame sequence for these receptors. B, Ethidium bromide staining of RT-PCR fragments. The mRNAs for both isoforms of the D2r were detected in a P7 brain sample, although no expression of this receptor was evidenced by RT-PCR in the glial samples at 7 or 14 DIV. The 1236 bp cDNA for the long D3r was readily amplified from the P7 brain sample. A single band with the same relative size was also detected in RNA samples from 7 and 14 DIV primary cultures of glia.
Fig. 2.
D3r mRNA is produced in oligodendroglial-like cells in culture. D3r message was detected in 14 DIV primary cultures of mouse glia by in situ hybridization using an antisense oligonucleotide labeled with digoxigenin specific for the 3′ end of exon 6 of D3r (B). Note that only cells with the morphology described for oligodendrocytes were detected. The staining is restricted to only the oligodendroglial cell bodies, and no message was evidenced within the cellular processes. The astrocytic layer (lining underneath the oligodendrocytes) was barely stained, and it is comparable to the nonspecific staining using a sense oligonucleotide (A). Scale bar, 20 μm.
Fig. 3.
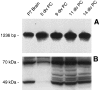
The in vitro expression of D3r seems to follow a developmentally regulated pattern. A, By Southern blot analysis using a specific radiolabeled internal oligonucleotide, D3r message was identified in RT-PCR samples generated from P7 brain and glial primary cultures at 5, 9, 11, and 14 DIV.B, By immunoblot detection, D3r proteins were detected in the membrane extracts from the olfactory tubercle isolated from P7 brain as a 49 kDa product (possibly the nonglycosylated form of the receptor) and a 70 kDa product (possibly the glycosylated form of the receptor). D3r proteins were also immunodetected in membrane proteins extracted from primary cultures at 5, 9, 11, and 14 DIV.
Fig. 4.
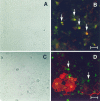
D3r colocalizes with A2B5+ but only occasionally with O1+ cells. Primary cultures were fixed at 7 DIV (A, B) and 14 DIV (C, D) and were analyzed by double immunofluorescence to detect D3r (green) with A2B5 (B) and O1 (D) (red). A, C, Phase-contrast micrographs corresponding to B and_D_, respectively. D3r colocalized with the early oligodendroglial marker A2B5 in all progenitor cells (B, arrows). At later stages in differentiation, oligodendrocytes that have started the synthesis of myelin membranes (evidenced with the monoclonal antibody O1; D) were faintly stained in their soma with the antibody against D3r (D). However, many D3r+ O1− cells without visible processes were also observed (D, arrows). Scale bars: B, 15 μm; D, 20 μm.
Fig. 5.
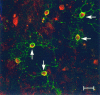
D3r was detected in association with the plasma membrane but not with the cellular processes in differentiating oligodendrocytes. The subcellular localization of the D3r was studied in double immunofluorescence (D3r, red; OO7,green) staining of 9 DIV primary cultures by confocal microscopy. D3r was detected in association with the plasma membrane of oligodendrocytes, which could be clearly evidenced with the OO7 antibody. D3r immunoreaction was also observed within the cytoplasm of these cells. A network of cellular processes was easily visualized with OO7; however, no D3r immunoreaction was detected in association with them. Scale bar, 20 μm.
Fig. 6.
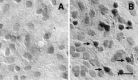
D3r was detected in differentiating oligodendrocytes in the corpus callosum. The anatomical distribution of D3r was examined by immunohistochemistry in brain sections during the first 4 postnatal weeks and in the adult brain. A, Nonspecific staining of P14 corpus callosum using nonimmune serum.B, Mesocorticolimbic large-sized neurons from Islands of Calleja were heavily immunostained with the anti-D3r antibody.C–E, Micrographs showing a portion of the genu corpus callosum immunostained with the anti-D3r antibody at P3 (C), P14 (D), and P25 (E). Cells with small somal diameters (∼7 μm) arranged in “strings” (arrowheads) or scattered individually throughout the white matter (arrows) were evident at P14 and to a lesser extent at P25. Scale bars:B, 20 mm; C–E, 10 mm.
Fig. 7.
Stimulation of D3r by quinpirole decreased the_in vitro_ differentiation of oligodendrocytes.A, The in vitro differentiation of oligodendrocytes was analyzed by counting the number of immature A2B5+ cells remaining in 14 DIV cultures and compared with the respective number in cultures at 7 DIV. The activation of D3r by quinpirole induced an increase of the number of A2B5+ cells in 14 DIV primary cultures with respect to the untreated control. This effect was blocked by coincubation with the antagonist haloperidol. Data are presented as the number of A2B5+ cells counted per field (0.1 mm2). At least 10 fields were observed from three separated experiments. Results were analyzed by the one-way ANOVA test (p < 0.05). The number of oligodendrocytes present at 14 DIV (analyzed by the presence of the marker O1) was also counted and analyzed as described previously. B,In vitro differentiation of oligodendrocytes was also evaluated by counting the number of O1+ cells connected to myelin-like membranes in 14 DIV (condition 1) and 16 DIV (condition 2) primary cultures. In condition 1, quinpirole or haloperidol treatments were begun at 6 DIV and continued until 14 DIV, and cultures were analyzed. Under this condition quinpirole induced a marked decrease (46%) of O1+ cells bearing myelin sheets compared with the untreated or haloperidol controls. In condition 2, quinpirole or haloperidol treatments were begun at 6 DIV, the drugs were removed at 12 DIV, and the cultures were analyzed at 16 DIV. Quinpirole treatment for day 6 appeared to be sufficient to reduce the numbers of sheet-bearing cells at 16 DIV with no apparent recovery after removal of the drug. Data represent the average of three separated experiments in triplicate and were analyzed by the one-way ANOVA test (p < 0.05). C, D, Immunofluorescence analysis of O1+ cells associated to myelin-like membranes in 14 DIV control (C) and quinpirole-treated (D) primary cultures. Observe the lesser extension of myelin-like membranes in the treated samples (D). Scale bar, 25 μm.
Similar articles
- Coexpression of dopamine D1 and D3 receptors in islands of Calleja and shell of nucleus accumbens of the rat: opposite and synergistic functional interactions.
Ridray S, Griffon N, Mignon V, Souil E, Carboni S, Diaz J, Schwartz JC, Sokoloff P. Ridray S, et al. Eur J Neurosci. 1998 May;10(5):1676-86. doi: 10.1046/j.1460-9568.1998.00173.x. Eur J Neurosci. 1998. PMID: 9751140 - Role of Dopamine D2/D3 Receptors in Development, Plasticity, and Neuroprotection in Human iPSC-Derived Midbrain Dopaminergic Neurons.
Bono F, Savoia P, Guglielmi A, Gennarelli M, Piovani G, Sigala S, Leo D, Espinoza S, Gainetdinov RR, Devoto P, Spano P, Missale C, Fiorentini C. Bono F, et al. Mol Neurobiol. 2018 Feb;55(2):1054-1067. doi: 10.1007/s12035-016-0376-3. Epub 2017 Jan 14. Mol Neurobiol. 2018. PMID: 28092083 - Ontogeny of glycerol phosphate dehydrogenase-positive oligodendrocytes in rat brain. Impaired differentiation of oligodendrocytes in the myelin deficient mutant rat.
Gordon MN, Kumar S, Espinosa de los Monteros A, de Vellis J. Gordon MN, et al. Int J Dev Neurosci. 1992 Aug;10(4):243-53. doi: 10.1016/0736-5748(92)90013-p. Int J Dev Neurosci. 1992. PMID: 1384273 - Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination.
Barateiro A, Fernandes A. Barateiro A, et al. Biochim Biophys Acta. 2014 Sep;1843(9):1917-29. doi: 10.1016/j.bbamcr.2014.04.018. Epub 2014 Apr 21. Biochim Biophys Acta. 2014. PMID: 24768715 Review. - Current drug treatments targeting dopamine D3 receptor.
Leggio GM, Bucolo C, Platania CB, Salomone S, Drago F. Leggio GM, et al. Pharmacol Ther. 2016 Sep;165:164-77. doi: 10.1016/j.pharmthera.2016.06.007. Epub 2016 Jun 22. Pharmacol Ther. 2016. PMID: 27343365 Review.
Cited by
- Prefrontal white matter impairment in substance users depends upon the catechol-o-methyl transferase (COMT) val158met polymorphism.
Zhang X, Lee MR, Salmeron BJ, Stein DJ, Hong LE, Geng X, Ross TJ, Li N, Hodgkinson C, Shen PH, Yang Y, Goldman D, Stein EA. Zhang X, et al. Neuroimage. 2013 Apr 1;69:62-9. doi: 10.1016/j.neuroimage.2012.11.056. Epub 2012 Dec 6. Neuroimage. 2013. PMID: 23219927 Free PMC article. - Interaction between Neurons and the Oligodendroglial Lineage in Multiple Sclerosis and Its Preclinical Models.
Pantazou V, Roux T, Oliveira Moreira V, Lubetzki C, Desmazières A. Pantazou V, et al. Life (Basel). 2021 Mar 11;11(3):231. doi: 10.3390/life11030231. Life (Basel). 2021. PMID: 33799653 Free PMC article. Review. - Antipsychotic drugs counteract autophagy and mitophagy in multiple sclerosis.
Patergnani S, Bonora M, Ingusci S, Previati M, Marchi S, Zucchini S, Perrone M, Wieckowski MR, Castellazzi M, Pugliatti M, Giorgi C, Simonato M, Pinton P. Patergnani S, et al. Proc Natl Acad Sci U S A. 2021 Jun 15;118(24):e2020078118. doi: 10.1073/pnas.2020078118. Proc Natl Acad Sci U S A. 2021. PMID: 34099564 Free PMC article. - Deficit of satellite oligodendrocytes of neurons in the rostral part of the head of the caudate nucleus in schizophrenia.
Kolomeets NS, Uranova NA. Kolomeets NS, et al. Eur Arch Psychiatry Clin Neurosci. 2025 Apr;275(3):813-822. doi: 10.1007/s00406-024-01869-x. Epub 2024 Jul 29. Eur Arch Psychiatry Clin Neurosci. 2025. PMID: 39073446 - COMT genotype affects prefrontal white matter pathways in children and adolescents.
Thomason ME, Dougherty RF, Colich NL, Perry LM, Rykhlevskaia EI, Louro HM, Hallmayer JF, Waugh CE, Bammer R, Glover GH, Gotlib IH. Thomason ME, et al. Neuroimage. 2010 Nov 15;53(3):926-34. doi: 10.1016/j.neuroimage.2010.01.033. Epub 2010 Jan 18. Neuroimage. 2010. PMID: 20083203 Free PMC article.
References
- Ariano MA, Sibley DR. Dopamine receptor distribution in the rat CNS: elucidation using anti-peptide antisera directed against D1A and D3 subtypes. Brain Res. 1994;649:95–110. - PubMed
- Barres BA, Schmid R, Sendtner M, Raff MC. Multiple extracellular signals are required for long-term oligodendrocyte survival. Development. 1993;118:283–295. - PubMed
- Bongarzone ER, Foster LM, Byravan S, Verity AN, Landry CF, Schonmann V, Amur-Umarjee S, Campagnoni AT. Conditionally immortalized neural cell lines: potential models for the study of neural cell function. In: Murphy S, editor. Methods, a companion to methods in enzymology. Academic; San Diego: 1996. pp. 489–500. - PubMed
- Bunzow JR, Van Tol HH, Grandy DK, Albert P, Salon J, Christie M, Machida CA, Neve KA, Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988;336:783–787. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases