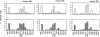Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection - PubMed (original) (raw)
Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection
D H Busch et al. J Exp Med. 1998.
Abstract
The mechanisms underlying the genesis and maintenance of T cell memory remain unclear. In this study, we examined the evolution of a complex, antigen-specific T cell population during the transition from primary effector to memory T cells after Listeria monocytogenes infection. T cell populations specific for listeriolysin O (LLO)91-99, the immunodominant epitope recognized by H2-Kd-restricted T lymphocytes, were directly identified in immune spleens using tetrameric H2-Kd-epitope complexes. The T cell receptor (TCR) Vbeta repertoire of specific T cells was determined by direct, ex vivo staining with a panel of mAbs. We demonstrate that LLO91-99-specific, primary effector T cell populations have a diverse TCR Vbeta repertoire. Analyses of memory T cell populations demonstrated similar TCR diversity. Furthermore, experiments with individual mice demonstrated that primary effector and memory T cells have indistinguishable TCR repertoires. Remarkably, after reinfection with L. monocytogenes, LLO91-99-specific T cells have a narrower TCR repertoire than do primary effector or memory T cells. Thus, our studies show that the TCR repertoire of primary effector T lymphocytes is uniformly transmitted to memory T cells, whereas expansion of memory T cells is selective.
Figures
Figure 1
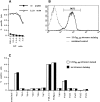
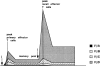
Folding and biotinylation of soluble H2-Kd and generation of tetramers. (A) The cDNA for murine H2-Kd was mutagenized by PCR to delete the leader sequence (LS), the transmembrane (TM), and the cytosolic domain (CD), and to extend the COOH terminus with a biotinylation sequence (indicated in single letter amino acid code) recognized by the E. coli BirA enzyme. The biotinylated lysine residue is enclosed in a box. (B) Recombinant H2-Kd and human β2m were overexpressed in E. coli and inclusion bodies were purified, resolubilized, and folded with LLO91–99 peptide as indicated in Materials and Methods. A typical fast protein liquid chromatography (FPLC) gel filtration absorbance profile demonstrates a large peak consisting of folded H2-Kd, β2m (seen in the gel inset), and peptide. HC indicates a small peak of aggregated, unfolded H2-Kd heavy chains. (C) Refolded, FPLC-purified, and biotinylated H2-Kd complexes were either directly subjected to PAGE (first labeled lane) or precipitated with conformation-dependent anti–H2-Kd-specific antibody SF1-1.1.1 (anti-Kd), control mouse IgG (mIgG) or streptavidin-agarose beads (SA). After SA precipitation, only a very small amount of folded H2-Kd could be precipitated with SF1-1.1.1 (anti-Kd, right lane). (D) Biotinylated H2-Kd complexes were mixed with streptavidin and again subjected to FPLC gel chromatography. The absorbance profile demonstrates a high molecular weight complex consisting of tetramerized H2-Kd–β2m–peptide complexes (gel inset shows H2-Kd heavy chain, β2m, and a faint band of streptavidin). The large peak consists of free streptavidin and carrier BSA (BSA/SA).
Figure 2
Costaining with LLO91–99–H2-Kd tetramers and TCR Vβ mAbs. A LLO91–99-specific CTL line was generated from an _L. monocytogenes_–immunized BALB/c mouse by in vitro peptide restimulation. (A) P815 (H2d) target cells were labeled with 51Cr and incubated in the presence (open circles) and absence (closed circles) of 10−6 M LLO91–99and decreasing numbers of LLO91–99-specific CTL. The percentage of specific lysis and the E/T ratio are indicated. (B) The CTL line was stained for CD8 (anti-CD8α Cy-Chrome) and LLO91–99 tetramers (PE-conjugated). Gating for CD8+ blasts revealed that nearly all T cell blasts stained with LLO91–99 tetramers. (C) Lymphoblasts were stained with a panel of FITC-conjugated, Vβ-specific antibodies in the presence (white bars) and absence (black bars) of LLO91–99 tetramers.
Figure 3
Direct ex vivo TCR staining of LLO91–99-specific T cells. CD8+ T cells from the spleen of a BALB/c mouse immunized 7 d previously with a sublethal dose of L. monocytogenes were stained with LLO91–99tetramers (PE-conjugated) and FITC-conjugated antibody specific for TCR-α/β (left) and the TCR Vβ8 chain (right).
Figure 4
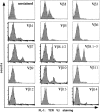
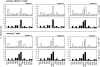
TCR Vβ staining reveals multiple subpopulations of LLO91– 99-specific T cells. Immune BALB/c CD8+ splenocytes obtained 7 d after_L. monocytogenes_ infection were stained with LLO91–99-specific tetramers and a panel of 14 different FITC-conjugated, Vβ-specific mAbs. These histograms demonstrate the proportion of cells that are stained with each of the antibodies.
Figure 6
Primary and memory, LLO91–99-specific T cells from individual mice have indistinguishable ratios of TCR Vβ chains. (top) Three BALB/c mice were immunized with a sublethal dose of L. monocytogenes and 7 d later peripheral blood lymphocytes were restimulated with LLO91–99-coated splenocytes. 10 d later these T cell lines were stained with LLO91–99 tetramers and the panel of TCR Vβ–specific antibodies (white bars; primary effector T cells). (bottom) 35 d after infection, these three BALB/c mice were killed and CD8+ T cells were isolated from spleens and stained with LLO91–99 tetramers and the panel of TCR Vβ–specific antibodies (hatched bars; memory T cells). The percentage of cells stained with each of the TCR antibodies is indicated.
Figure 7
After a recall response, LLO91–99-specific T cells express a more limited TCR repertoire than do primary effector T cells. Four BALB/c mice were infected with a sublethal dose of L. monocytogenes and 7 d later peripheral blood lymphocytes were used to generate LLO91–99-specific T cell lines, as described for Fig. 6. These T cell lines were stained with LLO91–99 tetramers and the panel of TCR Vβ-specific antibodies (white bars; primary effector T cells). 35 d after primary infection, these four BALB/c mice were reinfected with a 50-fold higher dose (100,000 bacteria) and 5 d later CD8+splenocytes were isolated and stained with LLO91–99 tetramers and the TCR Vβ panel (hatched bars; recall effector T cells). The percentage of cells stained with each of the TCR antibodies is indicated.
Figure 5
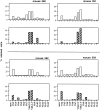
LLO91–99-specific primary and memory T cell repertoires closely reflect the general TCR repertoire of BALB/c CD8+ T cells. Six BALB/c mice were infected with a sublethal dose of L. monocytogenes, and CD8+ T cells from three mice were stained for TCR Vβ expression 7 d after infection (top, primary effector T cells) and from the remaining three mice 35 d after infection (bottom, memory T cells). White bars indicate the percentage of LLO91–99 tetramer-positive cells that stain with the individual TCR Vβ specific antibodies. Black bars indicate the percentage of overall CD8+ T cells that stain with the TCR Vβ-specific antibodies. Minimum number of gated CD8+ and tetramer-positive T cells for each TCR Vβ staining was 2,000 for primary effector T cells and 1,000 for memory T cells, respectively. n.d. = not done.
Figure 8
Model for TCR repertoire evolution during primary and recall infection with L. monocytogenes. The diversity of a pathogen-specific T cell population expanded during primary infection (arrows indicate time points of infection) is maintained in the memory pool. After rechallenge with the pathogen, the recall TCR repertoire is more restricted compared with the primary effector and memory T cell populations. These differences might be due to different in vivo expansion rates of T cells within the epitope-specific population.
Similar articles
- Intestinal and splenic T cell responses to enteric Listeria monocytogenes infection: distinct repertoires of responding CD8 T lymphocytes.
Huleatt JW, Pilip I, Kerksiek K, Pamer EG. Huleatt JW, et al. J Immunol. 2001 Mar 15;166(6):4065-73. doi: 10.4049/jimmunol.166.6.4065. J Immunol. 2001. PMID: 11238655 - Cytotoxic-T-lymphocyte responses to epitopes of listeriolysin O and p60 following infection with Listeria monocytogenes.
Bouwer HG, Hinrichs DJ. Bouwer HG, et al. Infect Immun. 1996 Jul;64(7):2515-22. doi: 10.1128/iai.64.7.2515-2522.1996. Infect Immun. 1996. PMID: 8698474 Free PMC article. - A novel approach of direct ex vivo epitope mapping identifies dominant and subdominant CD4 and CD8 T cell epitopes from Listeria monocytogenes.
Geginat G, Schenk S, Skoberne M, Goebel W, Hof H. Geginat G, et al. J Immunol. 2001 Feb 1;166(3):1877-84. doi: 10.4049/jimmunol.166.3.1877. J Immunol. 2001. PMID: 11160235 - MHC class I restricted T cell responses to Listeria monocytogenes, an intracellular bacterial pathogen.
Finelli A, Kerksiek KM, Allen SE, Marshall N, Mercado R, Pilip I, Busch DH, Pamer EG. Finelli A, et al. Immunol Res. 1999;19(2-3):211-23. doi: 10.1007/BF02786489. Immunol Res. 1999. PMID: 10493175 Review. - Acquired immunity to an intracellular pathogen: immunologic recognition of L. monocytogenes-infected cells.
Bouwer HG, Barry RA, Hinrichs DJ. Bouwer HG, et al. Immunol Rev. 1997 Aug;158:137-46. doi: 10.1111/j.1600-065x.1997.tb01000.x. Immunol Rev. 1997. PMID: 9314082 Review.
Cited by
- Selective expansion of cross-reactive CD8(+) memory T cells by viral variants.
Haanen JB, Wolkers MC, Kruisbeek AM, Schumacher TN. Haanen JB, et al. J Exp Med. 1999 Nov 1;190(9):1319-28. doi: 10.1084/jem.190.9.1319. J Exp Med. 1999. PMID: 10544203 Free PMC article. - Tracking phenotypically and functionally distinct T cell subsets via T cell repertoire diversity.
Kedzierska K, La Gruta NL, Stambas J, Turner SJ, Doherty PC. Kedzierska K, et al. Mol Immunol. 2008 Feb;45(3):607-18. doi: 10.1016/j.molimm.2006.05.017. Epub 2007 Aug 24. Mol Immunol. 2008. PMID: 17719639 Free PMC article. Review. - T cell costimulatory molecules in anti-viral immunity: Potential role in immunotherapeutic vaccines.
Watts TH, Bertram EM, Bukczynski J, Wen T. Watts TH, et al. Can J Infect Dis. 2003 Jul;14(4):221-9. doi: 10.1155/2003/214034. Can J Infect Dis. 2003. PMID: 18159461 Free PMC article. - Reverse TCR repertoire evolution toward dominant low-affinity clones during chronic CMV infection.
Schober K, Voit F, Grassmann S, Müller TR, Eggert J, Jarosch S, Weißbrich B, Hoffmann P, Borkner L, Nio E, Fanchi L, Clouser CR, Radhakrishnan A, Mihatsch L, Lückemeier P, Leube J, Dössinger G, Klein L, Neuenhahn M, Oduro JD, Cicin-Sain L, Buchholz VR, Busch DH. Schober K, et al. Nat Immunol. 2020 Apr;21(4):434-441. doi: 10.1038/s41590-020-0628-2. Epub 2020 Mar 16. Nat Immunol. 2020. PMID: 32205883 - Human leucocyte antigen-A2 restricted and Mycobacterium tuberculosis 19-kDa antigen-specific CD8+ T-cell responses are oligoclonal and exhibit a T-cell cytotoxic type 2 response cytokine-secretion pattern.
Höhn H, Kortsik C, Nilges K, Necker A, Freitag K, Tully G, Neukirch C, Maeurer MJ. Höhn H, et al. Immunology. 2001 Nov;104(3):278-88. doi: 10.1046/j.1365-2567.2001.01307.x. Immunology. 2001. PMID: 11722642 Free PMC article.
References
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science. 1996;272:54–60. - PubMed
- Sprent J. Immunological memory. Curr Opin Immunol. 1997;9:371–379. - PubMed
- Bruno L, Kirberg J, von Boehmer H. On the cellular basis of immunological memory. Immunity. 1995;2:37–43. - PubMed
- Pihlgren M, Lightstone L, Mamalaki C, Rimon G, Kioussis D. Expression in vivo of CD45RA, CD45RB and CD44 on T cell receptor–transgenic CD8+ T cells following immunization. Eur J Immunol. 1995;25:1755–1759. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases