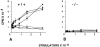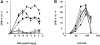Spontaneous skin ulceration and defective T cell function in CD18 null mice - PubMed (original) (raw)
. 1998 Jul 6;188(1):119-31.
doi: 10.1084/jem.188.1.119.
H Lu, K Norman, N van Nood, F Munoz, S Grabbe, M McArthur, I Lorenzo, S Kaplan, K Ley, C W Smith, C A Montgomery, S Rich, A L Beaudet
Affiliations
- PMID: 9653089
- PMCID: PMC2525537
- DOI: 10.1084/jem.188.1.119
Spontaneous skin ulceration and defective T cell function in CD18 null mice
K Scharffetter-Kochanek et al. J Exp Med. 1998.
Abstract
A null mutation was prepared in the mouse for CD18, the beta2 subunit of leukocyte integrins. Homozygous CD18 null mice develop chronic dermatitis with extensive facial and submandibular erosions. The phenotype includes elevated neutrophil counts, increased immunoglobulin levels, lymphadenopathy, splenomegaly, and abundant plasma cells in skin, lymph nodes, gut, and kidney. Very few neutrophils were found in spontaneously occurring skin lesions or with an induced toxic dermatitis. Intravital microscopy in CD18 null mice revealed a lack of firm neutrophil attachment to venules in the cremaster muscle in response to N-formyl- methionyl-leucyl-phenylalanine. A severe defect in T cell proliferation was found in the CD18 null mice when T cell receptors were stimulated either by staphylococcal enterotoxin A or by major histocompatibility complex alloantigens demonstrating a greater role of CD11/CD18 integrins in T cell responses than previously documented. The null mice are useful for delineating the functions of CD18 in vivo.
Figures
Figure 1
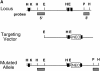
Preparation and analysis of the CD18 null mutation. A shows the genomic region to be targeted above with the targeted mutation below. DNA length is drawn to scale and the first three exons are indicated as solid rectangles. The location of the 3′ and 5′ flanking probes are shown as hatched boxes. Restriction enzyme sites are indicated as follows: E, EcoRI; H, HindIII; K, KpnI; and P, Pst. The targeting vector was linearized with KpnI, and a replacement mutation was obtained with insertion of a neomycin cassette at a PstI site that occurs precisely at the boundary of the intron and the 5′ end of exon 3. B shows a Southern blot with the 3′ flanking probe and genomic DNA isolated from the tails of wild-type (+/+), homozygous CD18 null (−/−), and heterozygous (+/−) mice. C presents the analysis of expression of CD18 on the surface of leukocytes from wild-type and homozygous CD18 null mice. FACS® analysis was performed as described in Materials and Methods. An isotype-matched control antibody was used (top). Expression of CD18 was quantitated by immunostaining with FITC-conjugated mAb C71/16.
Figure 1
Preparation and analysis of the CD18 null mutation. A shows the genomic region to be targeted above with the targeted mutation below. DNA length is drawn to scale and the first three exons are indicated as solid rectangles. The location of the 3′ and 5′ flanking probes are shown as hatched boxes. Restriction enzyme sites are indicated as follows: E, EcoRI; H, HindIII; K, KpnI; and P, Pst. The targeting vector was linearized with KpnI, and a replacement mutation was obtained with insertion of a neomycin cassette at a PstI site that occurs precisely at the boundary of the intron and the 5′ end of exon 3. B shows a Southern blot with the 3′ flanking probe and genomic DNA isolated from the tails of wild-type (+/+), homozygous CD18 null (−/−), and heterozygous (+/−) mice. C presents the analysis of expression of CD18 on the surface of leukocytes from wild-type and homozygous CD18 null mice. FACS® analysis was performed as described in Materials and Methods. An isotype-matched control antibody was used (top). Expression of CD18 was quantitated by immunostaining with FITC-conjugated mAb C71/16.
Figure 1
Preparation and analysis of the CD18 null mutation. A shows the genomic region to be targeted above with the targeted mutation below. DNA length is drawn to scale and the first three exons are indicated as solid rectangles. The location of the 3′ and 5′ flanking probes are shown as hatched boxes. Restriction enzyme sites are indicated as follows: E, EcoRI; H, HindIII; K, KpnI; and P, Pst. The targeting vector was linearized with KpnI, and a replacement mutation was obtained with insertion of a neomycin cassette at a PstI site that occurs precisely at the boundary of the intron and the 5′ end of exon 3. B shows a Southern blot with the 3′ flanking probe and genomic DNA isolated from the tails of wild-type (+/+), homozygous CD18 null (−/−), and heterozygous (+/−) mice. C presents the analysis of expression of CD18 on the surface of leukocytes from wild-type and homozygous CD18 null mice. FACS® analysis was performed as described in Materials and Methods. An isotype-matched control antibody was used (top). Expression of CD18 was quantitated by immunostaining with FITC-conjugated mAb C71/16.
Figure 2
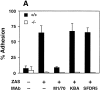
Analysis of neutrophil adherence and respiratory burst in wild-type and CD18 null mice. In A, adherence of murine neutrophils to a KLH-coated glass surface was measured as described in Materials and Methods. Isolated neutrophils were incubated with PBS or mAb for 30 min at room temperature: 10 μg/ml Ml/70 (anti-CD11b), 10 μg/ml KBA (anti-CD11a), or 10 μg/ml SFDR5 (Ig-matched control Ab). ZAS was added immediately before injecting the cell mixture into the adhesion chamber. Data are presented as mean ± SEM (n = 3); P <0.01 for wild-type (solid bars) versus CD18 null (open bars) in absence of antibodies. B depicts chemiluminescence of neutrophils from wild-type and CD18 null mice. Isolated neutrophils were mixed with 10−8 M luminal before the addition of PBS or 0.5 μg/ml of freshly opsonized zymosan particles (OpZ ). Result represents mean ± SEM (n = 3); P <0.01 for wild-type versus CD18 null with OpZ.
Figure 2
Analysis of neutrophil adherence and respiratory burst in wild-type and CD18 null mice. In A, adherence of murine neutrophils to a KLH-coated glass surface was measured as described in Materials and Methods. Isolated neutrophils were incubated with PBS or mAb for 30 min at room temperature: 10 μg/ml Ml/70 (anti-CD11b), 10 μg/ml KBA (anti-CD11a), or 10 μg/ml SFDR5 (Ig-matched control Ab). ZAS was added immediately before injecting the cell mixture into the adhesion chamber. Data are presented as mean ± SEM (n = 3); P <0.01 for wild-type (solid bars) versus CD18 null (open bars) in absence of antibodies. B depicts chemiluminescence of neutrophils from wild-type and CD18 null mice. Isolated neutrophils were mixed with 10−8 M luminal before the addition of PBS or 0.5 μg/ml of freshly opsonized zymosan particles (OpZ ). Result represents mean ± SEM (n = 3); P <0.01 for wild-type versus CD18 null with OpZ.
Figure 3
Gross morphology and histology of CD18 null mice. A demonstrates alopecia in the neck and upper thorax with severe ulcerative dermatitis and perioral soft tissue swelling. B shows extended facial alopecia and erosions with conjunctivitis of the eye. C demonstrates ulcerative dermatitis with full thickness necrosis of the epidermis covered by an eosinophilic crust with entrapped inflammatory cells and bacterial colonies (arrows). There is adjacent epidermal hyperplasia. The dermal inflammatory infiltrate consists of lymphocytes, histiocytes, mast cells, occasional plasma cells, and few neutrophils. E, epidermis; D, dermis; and U, ulceration. Original magnification 250×. The spleen is shown in D with hyperplasia of the red pulp, multiple plasma cells (*), and occasional Russell bodies (arrows), the latter indicative of abnormal deposition of immunoglobulins within plasma cells. Original magnification 400×.
Figure 3
Gross morphology and histology of CD18 null mice. A demonstrates alopecia in the neck and upper thorax with severe ulcerative dermatitis and perioral soft tissue swelling. B shows extended facial alopecia and erosions with conjunctivitis of the eye. C demonstrates ulcerative dermatitis with full thickness necrosis of the epidermis covered by an eosinophilic crust with entrapped inflammatory cells and bacterial colonies (arrows). There is adjacent epidermal hyperplasia. The dermal inflammatory infiltrate consists of lymphocytes, histiocytes, mast cells, occasional plasma cells, and few neutrophils. E, epidermis; D, dermis; and U, ulceration. Original magnification 250×. The spleen is shown in D with hyperplasia of the red pulp, multiple plasma cells (*), and occasional Russell bodies (arrows), the latter indicative of abnormal deposition of immunoglobulins within plasma cells. Original magnification 400×.
Figure 3
Gross morphology and histology of CD18 null mice. A demonstrates alopecia in the neck and upper thorax with severe ulcerative dermatitis and perioral soft tissue swelling. B shows extended facial alopecia and erosions with conjunctivitis of the eye. C demonstrates ulcerative dermatitis with full thickness necrosis of the epidermis covered by an eosinophilic crust with entrapped inflammatory cells and bacterial colonies (arrows). There is adjacent epidermal hyperplasia. The dermal inflammatory infiltrate consists of lymphocytes, histiocytes, mast cells, occasional plasma cells, and few neutrophils. E, epidermis; D, dermis; and U, ulceration. Original magnification 250×. The spleen is shown in D with hyperplasia of the red pulp, multiple plasma cells (*), and occasional Russell bodies (arrows), the latter indicative of abnormal deposition of immunoglobulins within plasma cells. Original magnification 400×.
Figure 3
Gross morphology and histology of CD18 null mice. A demonstrates alopecia in the neck and upper thorax with severe ulcerative dermatitis and perioral soft tissue swelling. B shows extended facial alopecia and erosions with conjunctivitis of the eye. C demonstrates ulcerative dermatitis with full thickness necrosis of the epidermis covered by an eosinophilic crust with entrapped inflammatory cells and bacterial colonies (arrows). There is adjacent epidermal hyperplasia. The dermal inflammatory infiltrate consists of lymphocytes, histiocytes, mast cells, occasional plasma cells, and few neutrophils. E, epidermis; D, dermis; and U, ulceration. Original magnification 250×. The spleen is shown in D with hyperplasia of the red pulp, multiple plasma cells (*), and occasional Russell bodies (arrows), the latter indicative of abnormal deposition of immunoglobulins within plasma cells. Original magnification 400×.
Figure 4
Increased bacteremia and mortality in CD18 null mice inoculated with S. pneumoniae. Mice were injected intraperitoneally with S. pneumoniae as described in Materials and Methods. Significant increased mortality (P = 0.0003; solid bars) and increased bacteremia (hatched bars) in surviving mice (P = <0.0001) were found for CD18 null mice.
Figure 5
Neutrophil response to toxic dermatitis. Ears were challenged with topical application of 10 μl 2% DNFB 5 h before death. The dense neutrophil infiltrate in a wild-type mouse is shown in A and the lack of neutrophil response despite the presence of edematous interstitial tissue is shown in a CD18 null mouse in B. Hematoxylin and eosin staining at an original magnification of 400× is shown. Dermis (d) and cartilage (c) are labeled.
Figure 5
Neutrophil response to toxic dermatitis. Ears were challenged with topical application of 10 μl 2% DNFB 5 h before death. The dense neutrophil infiltrate in a wild-type mouse is shown in A and the lack of neutrophil response despite the presence of edematous interstitial tissue is shown in a CD18 null mouse in B. Hematoxylin and eosin staining at an original magnification of 400× is shown. Dermis (d) and cartilage (c) are labeled.
Figure 6
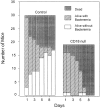
Firm attachment and rolling of leukocytes is altered in CD18 null mice. Intravital microscopy was performed as described in Materials and Methods. Venules with diameters between 20 and 40 μm were observed and recorded through a CCD camera. Almost no firm attachment of leukocyte occurred in a wild-type mouse before fMLP infusion (A). Infusion of fMLP resulted in a substantial increase in firmly attached leukocytes in a wild-type mouse (C ). No firm attachment of leukocytes to the endothelial lining was observed in CD18 null mice before or after fMLP stimulation (B and D, respectively). The number of firmly adherent leukocytes in the observed vessels was quantitated before fMLP infusion and 1 min after infusion (E). Results are presented as number of firmly adherent cells/100 μm of vessel length. Rolling leukocyte fluxes were determined before fMLP infusion and 1 min after infusion (F ). Results (mean ± SEM, n = 3) are expressed as the flux of rolling leukocytes per minute.
Figure 7
Abrogation of mixed lymphocyte reaction in CD18 null mice. Splenocytes from three wild-type littermate controls (A) and from three CD18 null mice (B) were stimulated in a mixed lymphocyte reaction. Responder cells (4 × 105 cells per well) were mixed with increasing concentrations of allogeneic BALB/c (filled symbols) or with syngeneic wild-type or CD18 null spleen cells that had been irradiated (open symbols). Analysis of proliferation was performed after 96 h in culture as described in Materials and Methods. Data are presented as mean cpm ± SEM of triplicate cultures.
Figure 8
Abrogation of T cell proliferation in response to staphylococcal enterotoxin A (SEA) in CD18 null mice. In A, splenocytes from three wild-type littermates (filled symbols) and from CD18 null mice (open symbols) were cultured without addition or with 0.1 ng to 20 μg/ml SEA. In B, splenocytes from wild-type (filled symbols) or CD18 null (open symbols) mice were stimulated with 100 ng/ml phorbol myristate acetate and 200 ng/ml ionomycin and analyzed for proliferation at the indicated times. Proliferation was quantitated as described in Materials and Methods and is expressed as mean cpm ± SEM of triplicate cultures for three mice of each genotype.
Similar articles
- Neutrophil emigration in the skin, lungs, and peritoneum: different requirements for CD11/CD18 revealed by CD18-deficient mice.
Mizgerd JP, Kubo H, Kutkoski GJ, Bhagwan SD, Scharffetter-Kochanek K, Beaudet AL, Doerschuk CM. Mizgerd JP, et al. J Exp Med. 1997 Oct 20;186(8):1357-64. doi: 10.1084/jem.186.8.1357. J Exp Med. 1997. PMID: 9334375 Free PMC article. - Molecular Insights Into Neutrophil Biology From the Zebrafish Perspective: Lessons From CD18 Deficiency.
Bader A, Gao J, Rivière T, Schmid B, Walzog B, Maier-Begandt D. Bader A, et al. Front Immunol. 2021 Sep 7;12:677994. doi: 10.3389/fimmu.2021.677994. eCollection 2021. Front Immunol. 2021. PMID: 34557186 Free PMC article. - Impairment of neutrophil emigration in CD18-null mice.
Walzog B, Scharffetter-Kochanek K, Gaehtgens P. Walzog B, et al. Am J Physiol. 1999 May;276(5):G1125-30. doi: 10.1152/ajpgi.1999.276.5.G1125. Am J Physiol. 1999. PMID: 10330002 - Intracellular β2 integrin (CD11/CD18) interacting partners in neutrophil trafficking.
Thome S, Begandt D, Pick R, Salvermoser M, Walzog B. Thome S, et al. Eur J Clin Invest. 2018 Nov;48 Suppl 2:e12966. doi: 10.1111/eci.12966. Epub 2018 Jun 29. Eur J Clin Invest. 2018. PMID: 29896791 Review. - CD18 in monogenic and polygenic inflammatory processes of the skin.
Peters T, Sindrilaru A, Wang H, Oreshkova T, Renkl AC, Kess D, Scharffetter-Kochanek K. Peters T, et al. J Investig Dermatol Symp Proc. 2006 Sep;11(1):7-15. doi: 10.1038/sj.jidsymp.5650006. J Investig Dermatol Symp Proc. 2006. PMID: 17069006 Review.
Cited by
- Tracing conidial fate and measuring host cell antifungal activity using a reporter of microbial viability in the lung.
Jhingran A, Mar KB, Kumasaka DK, Knoblaugh SE, Ngo LY, Segal BH, Iwakura Y, Lowell CA, Hamerman JA, Lin X, Hohl TM. Jhingran A, et al. Cell Rep. 2012 Dec 27;2(6):1762-73. doi: 10.1016/j.celrep.2012.10.026. Epub 2012 Nov 29. Cell Rep. 2012. PMID: 23200858 Free PMC article. - Isoflurane inhibits neutrophil recruitment in the cutaneous Arthus reaction model.
Carbo C, Yuki K, Demers M, Wagner DD, Shimaoka M. Carbo C, et al. J Anesth. 2013 Apr;27(2):261-8. doi: 10.1007/s00540-012-1508-1. Epub 2012 Oct 25. J Anesth. 2013. PMID: 23096126 Free PMC article. - Sepsis pathophysiology and anesthetic consideration.
Yuki K, Murakami N. Yuki K, et al. Cardiovasc Hematol Disord Drug Targets. 2015;15(1):57-69. doi: 10.2174/1871529x15666150108114810. Cardiovasc Hematol Disord Drug Targets. 2015. PMID: 25567335 Free PMC article. Review. - Deciphering Tumor Cell Evolution in Cutaneous T-Cell Lymphomas: Distinct Differentiation Trajectories in Mycosis Fungoides and Sézary Syndrome.
Jiang TT, Cao S, Kruglov O, Virmani A, Geskin LJ, Falo LD Jr, Akilov OE. Jiang TT, et al. J Invest Dermatol. 2024 May;144(5):1088-1098. doi: 10.1016/j.jid.2023.10.018. Epub 2023 Nov 28. J Invest Dermatol. 2024. PMID: 38036289 - CD18 is required for optimal lymphopenia-induced proliferation of mouse T cells.
Sarin R, Abraham C. Sarin R, et al. Am J Physiol Gastrointest Liver Physiol. 2012 Oct;303(7):G851-60. doi: 10.1152/ajpgi.00520.2011. Epub 2012 Jul 19. Am J Physiol Gastrointest Liver Physiol. 2012. PMID: 22821945 Free PMC article.
References
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. - PubMed
- Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. - PubMed
- Danilenko DM, Rossitto PV, Van der Vieren M, Trong HL, McDonough SP, Affolter VK, Moore PF. A novel canine leukointegrin, αdβ2, is expressed by specific macrophage subpopulations in tissue and a minor CD8+lymphocyte subpopulation in peripheral blood. J Immunol. 1995;155:35–44. - PubMed
- Anderson DC, Springer TA. Leukocyte adhesion deficiency: an inherited defect in the Mac-1, LFA-1, and p150,95 glycoproteins. Annu Rev Med. 1987;38:175–194. - PubMed
- Arnaout MA. Leukocyte adhesion molecules deficiency: its structural basis, pathophysiology and implications for modulating the inflammatory response. Immunol Rev. 1990;114:145–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI30036/AI/NIAID NIH HHS/United States
- R37 AI032177/AI/NIAID NIH HHS/United States
- GM15483/GM/NIGMS NIH HHS/United States
- AI32177/AI/NIAID NIH HHS/United States
- N01AI30036/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials