The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha - PubMed (original) (raw)
The tyrosine kinase regulator Cbl enhances the ubiquitination and degradation of the platelet-derived growth factor receptor alpha
S Miyake et al. Proc Natl Acad Sci U S A. 1998.
Abstract
The Cbl protooncogene product has emerged as a negative regulator of receptor and nonreceptor tyrosine kinases. We recently demonstrated that oncogenic Cbl mutants upregulate the endogenous tyrosine kinase signaling machinery when expressed in the NIH 3T3 cells, and identified the platelet-derived growth factor receptor-alpha (PDGFRalpha) as one of the tyrosine kinases targeted by these oncogenes. These findings suggested a role for the normal Cbl protein in negative regulation of the PDGFRalpha. However, the mechanism of such negative regulation remained to be determined. Here we show that overexpression of the wild-type Cbl enhances the ligand-induced ubiquitination of the PDGFRalpha. Concomitantly, the PDGFRalpha in Cbl-overexpressing cells undergoes a faster ligand-induced degradation compared with that in the control cells. These results identify a role for Cbl in the regulation of ligand-induced ubiquitination and degradation of receptor tyrosine kinases and suggest one potential mechanism for evolutionarily conserved negative regulatory influence of Cbl on tyrosine kinases.
Figures
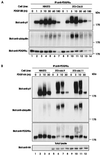
Figure 1
Ubiquitination of the PDGFRα upon PDGF stimulation of parental and Cbl-transfected NIH 3T3 cells. (A) Serum-starved parental (NIH 3T3) and Cbl-overexpressing NIH 3T3 cells (3T3-Cbl.8) were stimulated with 20 ng/ml human recombinant PDGF-BB and lysed at the indicated times (m, minutes). Immunoprecipitations (IP) were performed with an anti-PDGFRα antibody and 1 mg aliquots of each lysate, and were analyzed by anti-pY immunoblotting (Top). The membrane was stripped and serially reprobed with anti-ubiquitin (Middle) and anti-PDGFRα antibodies (Bottom). (B) Serum-starved parental (NIH 3T3) and Cbl-overexpressing NIH 3T3 cells (3T3-Cbl.9 and 3T3-Cbl.11) were stimulated with 20 ng/ml human recombinant PDGF-AA and lysed at the indicated times (m, minutes). Immunoprecipitation and immunoblotting with anti-pY (Top), anti-ubiquitin (Upper Middle) and anti-PDGFRα antibodies (Lower Middle) were performed as in A. Total lysates collected at each time point were immunoblotted with an anti-HA antibody (Bottom).
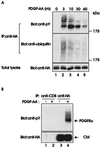
Figure 2
Association of Cbl with the ubiquitinated PDGFRα. (A) Serum-starved Cbl-expressing cells were stimulated with 20 ng/ml human recombinant PDGF-AA and lysed at the indicated times (m, minutes). Immunoprecipitations (IP) were carried out by using an anti-HA antibody (12CA5) and 1 mg of each lysate, followed by immunoblotting with an anti-pY antibody (Top). The membrane was stripped and reprobed with an anti-ubiquitin antibody (Middle). Total lysates collected at each time point were immunoblotted with anti-HA antibody (Bottom). (B) Lysates of unstimulated 3T3-Cbl.8 cells (−) and stimulated with 20 ng/ml PDGF-AA for 3 min (+) were immunoprecipitated with an anti-CD8 control antibody or anti-HA antibody followed by anti-pY immunoblotting (Upper). The membrane was stripped and reprobed with an anti-HA antibody (Lower).
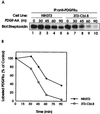
Figure 3
Time course of the ligand-induced degradation of the surface-labeled PDGFRα in parental and Cbl-transfected NIH 3T3 cells. Serum-starved parental NIH 3T3 and Cbl-expressing cells were surface labeled with biotin. Labeled cells were incubated either without (0) or with PDGF-AA for 15 min at 4°C. Cells were washed, incubated at 37°C for the indicated times, and lysed. Anti-PDGFRα immunoprecipitates of the lysates (1 mg) were subjected to blotting with avidin-HRP followed by enhanced chemiluminescence detection. (A) A representative blot. (B) Densitometry was performed and the intensity of PDGFRα signal at various time points was expressed as a percentage of that in unstimulated cells at time zero. •, PDGF-AA-stimulated NIH 3T3 cells; □, PDGF-AA-stimulated 3T3-Cbl.8 cells.
References
- Hunter T. Cell. 1995;80:225–236. - PubMed
- Tonks N K, Neel B G. Cell. 1996;87:365–368. - PubMed
- van der Geer P, Hunter T, Lindberg R A. Annu Rev Cell Biol. 1994;10:251–337. - PubMed
- Seaman M N, Burd C G, Emr S D. Curr Opin Cell Biol. 1996;8:549–556. - PubMed
- Langdon W Y. Aust NZ J Med. 1995;25:859–864. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous