Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia - PubMed (original) (raw)
Tissue factor transcription driven by Egr-1 is a critical mechanism of murine pulmonary fibrin deposition in hypoxia
S F Yan et al. Proc Natl Acad Sci U S A. 1998.
Abstract
Local hypoxemia and stasis trigger thrombosis. We have demonstrated previously that in a murine model of normobaric hypoxia pulmonary fibrin deposition is a result of expression of tissue factor, especially in oxygen-deprived mononuclear phagocytes (MPs). We now show that transcription factor early-growth-response gene product (Egr-1) is rapidly activated in hypoxia, both in vitro and in vivo, and is responsible for transcription and expression of tissue factor in hypoxic lung. MPs and HeLa cells subjected to hypoxia (pO2 approximately 13 torr) had increased levels of tissue factor transcripts (approximately 18-fold) and an increased rate of transcription (approximately 15-fold), based on nuclear run-on analysis. Gel-shift analysis of nuclear extracts from hypoxic MPs and HeLa cells demonstrated increased DNA-binding activity at the serum response region (SRR; -111/+14 bp) of the tissue factor promoter at Egr-1 motifs. Using 32P-labeled Egr consensus oligonucleotide, we observed induction of DNA-binding activity in nuclear extracts from hypoxic lung and HeLa cells because of activation of Egr-1, by means of supershift analysis. Transient transfection of HeLa cells with chimeric plasmids containing wild-type or mutant SRR from the tissue factor promoter showed that intact Sp1 sites are necessary for basal promoter activity, whereas the integrity of Egr-1 sites was required for hypoxia-enhanced expression. A central role for Egr-1 in hypoxia-mediated tissue factor expression was confirmed by experiments with homozygous Egr-1 null mice; wild-type mice subjected to oxygen deprivation expressed tissue factor and showed fibrin deposition, but hypoxic homozygous Egr-1 null mice displayed neither tissue factor nor fibrin. These data delineate a novel biology for hypoxia-induced fibrin deposition, in which oxygen deprivation-induced activation of Egr-1, resulting in expression of tissue factor, has an unexpected and central role.
Figures
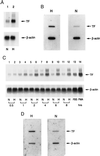
Figure 1
Effect of hypoxia on tissue factor gene expression in mononuclear phagocytes (A and B) and HeLa cells (C and D). (A and C) Northern analysis for tissue factor transcripts. Human peripheral blood mononuclear phagocytes (≈106 cells, A) or HeLa cells (≈0.5 × 106 cells, C) in serum-free medium were subjected to normoxia (N) or hypoxia (H; pO2 ≈ 12–14 torr) for the indicated times (HeLa) or for 4 hr (mononuclear phagocytes). Northern analysis was performed by loading 30 μg/lane of total RNA and using 32P-labeled cDNA for human tissue factor (Upper) or human β-actin (Lower). FBS and PMA denote cultures exposed to fetal bovine serum (20%) or phorbol myristate acetate (50 ng/ml), respectively, for 1 hr in each case. (B and D) Nuclear run-on analysis for the rate of tissue factor transcription. Mononuclear phagocytes (≈107 cells, B) or HeLa cells (≈106 cells, D) were subjected to normoxia or hypoxia (as above) for 4 hr, nuclei were harvested, labeled by incubation with [α-32P]dUTP for 1 hr, the RNA was isolated, and the same amount of radioactivity was hybridized with cDNA probes for tissue factor or β-actin, the latter already immobilized on nitrocellulose membranes. Results shown are representative of a minimum of four experiments.
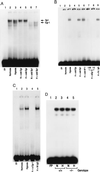
Figure 2
EMSA of tissue factor promoter DNA-binding motifs: effect of hypoxia. (A) EMSA using R2 from the tissue factor promoter. Rat mononuclear phagocytes (≈107, A) were exposed to normoxia (N) or hypoxia (H; pO2 ≈ 12–14 torr) for 45 min, nuclear extracts were prepared, and EMSA was performed using 32P-labeled oligonucleotide for R2 (−96/−66 bp; ref. 9). Each lane received 10 μg/lane of total nuclear extract protein. The arrows indicate bands corresponding to Egr and Sp1 (A). Note that the unlabeled lower band observed in lanes 2, 3, and 7 may represent Sp3 (38). A 100-fold excess of the indicated unlabeled (cold) oligonucleotide with a consensus sequence of Sp1 or Egr was added. (B) EMSA using consensus oligonucleotide probe Egr. Nuclear extracts were harvested from normoxic/hypoxic HeLa cells and EMSA was performed with the indicated 32P-labeled oligonucleotide probe. Excess unlabeled Sp1, AP-1, or Egr oligonucleotide was added to certain lanes, and either rabbit anti-Egr-1 IgG (1:5 and 1:50 dilution) or nonimmune IgG (1:5) was added to other lanes. (C) EMSA using consensus oligonucleotide for Egr, as above, and nuclear extracts from normoxic/hypoxic murine lung. Mice were subjected to hypoxia (≈6% oxygen; ref. 6) for 45 min, lungs were rapidly harvested, and nuclear extracts were prepared. EMSA was performed as above, and, where indicated, anti-Egr-1 IgG or nonimmune IgG (Santa Cruz Biotechnology) was added. (D) EMSA using consensus oligonucleotide for Sp-1 and nuclear extracts from normoxic/hypoxic murine lung was performed using the same conditions as above (+/+, wild-type mice; −/−, Egr-1 null mice). Results shown are representative of a minimum of four experiments.
Figure 3
Hypoxia-inducible tissue factor expression results from transcriptional activation at Egr-1 sites. (A) Transient cotransfection of HeLa cells was performed by using either pTF(−111/+14)Luc, pTF(EGR-1 m)Luc, pTF(SP1 m)Luc, or pTF(EGR-1 m/SP1 m)Luc, and pCMV-β-galactosidase. Cultures were transfected with each of the indicated constructs by using the lipofectamine procedure (GIBCO), and then cells were exposed to normoxia (N) or hypoxia (H) for 5 hr. Luciferase and β-galactosidase activity were then determined. Relative luciferase activity is luciferase activity normalized for β-galactosidase activity. (B) Transient transfection of HeLa cells using pYSF60 (consensus Egr wild-type sequence) or pYSF61 (mutationally inactivated Egr sequence) and pCMV-β-galactosidase by the same procedure described above. Results shown are representative of a minimum of four experiments.
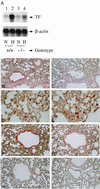
Figure 4
Hypoxia-mediated induction of tissue factor expression in murine lung: Egr-1 null mice show reduced tissue factor mRNA (A) and antigen (B_–_I). (A) Mice (wild-type, +/+, or homozygous null mice, −/−) were subjected to normoxia (N) or hypoxia (H; 6% oxygen), lungs were rapidly harvested, total RNA was prepared, and Northern analysis (30 μg/lane of total RNA) was performed with 32P-labeled cDNA for mouse tissue factor (Upper) or β-actin (Lower). (B_–_I) Immunohistochemical analysis for tissue factor antigen was performed on lung tissue from mice exposed to normoxia or hypoxia. (B and C) Hypoxic and normoxic wild-type mouse lung, respectively, stained with antitissue factor IgG. (D and E) Higher-power micrograph of hypoxic wild-type mouse lung stained with anti-tissue factor IgG (D) with an adjacent section stained with antibody to F4/80 to detect mononuclear phagocytes (E). (F and G) Higher-power micrograph of hypoxic wild-type mouse lung stained with anti-tissue factor IgG (F) with an adjacent section stained with antibody to smooth muscle α-actin (G). (H and I) Hypoxic and normoxic egr-1 −/− mouse stained with anti-tissue factor IgG. [×200 (B and C, F_–_I) and ×600 (D and E).] Results shown are representative of a minimum of three experiments.
Figure 5
Hypoxia-mediated induction of fibrin deposition in murine lung: egr-1 null mice show no increase in fibrin deposition. Wild-type or homozygous Egr-1 null mice were subjected to hypoxia as above. (A_–_D) Immunostaining was performed with antibody to fibrin γ-γ chain crosslinks (15). (A) Hypoxic wild-type murine lung. (B) Normoxic wild-type murine lung. (C) Hypoxic Egr-1 −/− murine lung. (D) Normoxic Egr-1 −/− murine lung. (×200.) (E) Immunoblotting (5) using the antibody to γ-γ chain crosslinks (15) was performed on plasmin-treated extracts of murine lung harvested from normoxic (N)/hypoxic (H) wild-type or Egr-1 −/− mice. Results shown are representative of a minimum of three experiments.
Similar articles
- Protein kinase C-beta and oxygen deprivation. A novel Egr-1-dependent pathway for fibrin deposition in hypoxemic vasculature.
Yan SF, Lu J, Zou YS, Kisiel W, Mackman N, Leitges M, Steinberg S, Pinsky D, Stern D. Yan SF, et al. J Biol Chem. 2000 Apr 21;275(16):11921-8. doi: 10.1074/jbc.275.16.11921. J Biol Chem. 2000. PMID: 10766820 - Hypoxia-associated induction of early growth response-1 gene expression.
Yan SF, Lu J, Zou YS, Soh-Won J, Cohen DM, Buttrick PM, Cooper DR, Steinberg SF, Mackman N, Pinsky DJ, Stern DM. Yan SF, et al. J Biol Chem. 1999 May 21;274(21):15030-40. doi: 10.1074/jbc.274.21.15030. J Biol Chem. 1999. PMID: 10329706 - Transcriptional regulation of the tissue factor gene in human epithelial cells is mediated by Sp1 and EGR-1.
Cui MZ, Parry GC, Oeth P, Larson H, Smith M, Huang RP, Adamson ED, Mackman N. Cui MZ, et al. J Biol Chem. 1996 Feb 2;271(5):2731-9. doi: 10.1074/jbc.271.5.2731. J Biol Chem. 1996. PMID: 8576248 - Hypoxia/Hypoxemia-Induced activation of the procoagulant pathways and the pathogenesis of ischemia-associated thrombosis.
Yan SF, Mackman N, Kisiel W, Stern DM, Pinsky DJ. Yan SF, et al. Arterioscler Thromb Vasc Biol. 1999 Sep;19(9):2029-35. doi: 10.1161/01.atv.19.9.2029. Arterioscler Thromb Vasc Biol. 1999. PMID: 10479642 Review. - The EGR family of transcription-regulatory factors: progress at the interface of molecular and systems neuroscience.
O'Donovan KJ, Tourtellotte WG, Millbrandt J, Baraban JM. O'Donovan KJ, et al. Trends Neurosci. 1999 Apr;22(4):167-73. doi: 10.1016/s0166-2236(98)01343-5. Trends Neurosci. 1999. PMID: 10203854 Review.
Cited by
- The molecular basis for the prothrombotic state in sickle cell disease.
Shet AS, Lizarralde-Iragorri MA, Naik RP. Shet AS, et al. Haematologica. 2020 Oct 1;105(10):2368-2379. doi: 10.3324/haematol.2019.239350. Haematologica. 2020. PMID: 33054077 Free PMC article. - Egr-1: is it always immediate and early?
Yan SF, Pinsky DJ, Mackman N, Stern DM. Yan SF, et al. J Clin Invest. 2000 Mar;105(5):553-4. doi: 10.1172/JCI9513. J Clin Invest. 2000. PMID: 10712422 Free PMC article. No abstract available. - Impact of Tissue Factor Localization on Blood Clot Structure and Resistance under Venous Shear.
Govindarajan V, Zhu S, Li R, Lu Y, Diamond SL, Reifman J, Mitrophanov AY. Govindarajan V, et al. Biophys J. 2018 Feb 27;114(4):978-991. doi: 10.1016/j.bpj.2017.12.034. Biophys J. 2018. PMID: 29490257 Free PMC article. - PKCbeta regulates ischemia/reperfusion injury in the lung.
Fujita T, Asai T, Andrassy M, Stern DM, Pinsky DJ, Zou YS, Okada M, Naka Y, Schmidt AM, Yan SF. Fujita T, et al. J Clin Invest. 2004 Jun;113(11):1615-23. doi: 10.1172/JCI19225. J Clin Invest. 2004. PMID: 15173888 Free PMC article. - Vascular smooth muscle cells express the transcriptional corepressor NAB2 in response to injury.
Silverman ES, Khachigian LM, Santiago FS, Williams AJ, Lindner V, Collins T. Silverman ES, et al. Am J Pathol. 1999 Oct;155(4):1311-7. doi: 10.1016/S0002-9440(10)65233-9. Am J Pathol. 1999. PMID: 10514413 Free PMC article.
References
- Geerts W, Code K, Jay R, Chen E, Szalai J. N Engl J Med. 1994;331:1601–1606. - PubMed
- Anderson F, Wheeler H, Goldberg R, Hosmer D, Patwardhan N, Jovanovic B, Forcier A, Dalen J. Arch Intern Med. 1991;151:933–938. - PubMed
- Malone P, Morris C. J Pathol. 1978;125:119–129. - PubMed
- Hamer J, Malone P, Silver I. Br J Surg. 1981;68:166–170. - PubMed
- Malone P. Med Hypotheses. 1977;5:189–201. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HD013063/HD/NICHD NIH HHS/United States
- P50 HD013063/HD/NICHD NIH HHS/United States
- HL35246/HL/NHLBI NIH HHS/United States
- R01 HL055397/HL/NHLBI NIH HHS/United States
- HL42507/HL/NHLBI NIH HHS/United States
- HL55397/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources