Identification of a cytotoxic T-lymphocyte response to the novel BARF0 protein of Epstein-Barr virus: a critical role for antigen expression - PubMed (original) (raw)
Identification of a cytotoxic T-lymphocyte response to the novel BARF0 protein of Epstein-Barr virus: a critical role for antigen expression
N Kienzle et al. J Virol. 1998 Aug.
Abstract
The Epstein-Barr virus (EBV)-encoded BARF0 open reading frame gene products are consistently expressed in EBV-positive Burkitt's lymphoma (BL) cell lines, nasopharyngeal carcinoma cell lines, and lymphoblastoid cell lines (LCLs). Here we show that the BARF0 sequence includes an HLA A*0201-restricted cytotoxic T-lymphocyte (CTL) epitope. By using theoretically predicted HLA A2 binding motifs and peptide-loaded antigen presentation-deficient T2 cells, polyclonal BARF0-specific CD8(+) CTLs were isolated from four different healthy EBV-seropositive donors but not from two seronegative donors. These CTL lines recognized the peptide epitope LLWAARPRL, which was found to be conserved in 33 of 34 virus strains originating from Caucasian, African, and Asian individuals. The BARF0-specific CTL lines could lyse EBV-negative BL cells stably transfected with the BARF0 gene but did not kill HLA A2-matched EBV-positive BL cells and LCLs in a standard 51Cr release assay. Reverse transcriptase PCR analysis demonstrated that these EBV-positive cell lines expressed significantly lower levels of BARF0 mRNA than transfected cells. This data indicated that the BARF0 epitope could be endogenously processed; however, antigen levels in the target cell were a limiting factor for the effective interaction between BARF0-expressing cells and CTLs. The limited expression of BARF0 antigen in EBV-infected BL cells and LCLs might contribute to the escape of immune recognition from virus-specific CTLs present in the host.
Figures
FIG. 1
HLA A2 stabilization analysis with T2 cells. T2 cells were either untreated (no peptide) or incubated with BARF0 peptide (pep.) 130 (PPRARDRA
LLWAARPRL
LLS) or 131 (WAARP
RLLLSLQQV
PEPRLA). For a positive control (pos. contr.), peptide YLLEMLWRL from LMP-1 was used. HLA A2 expression on these cells was then analyzed by using a FACSscan and an HLA A2-specific MAb.
FIG. 2
Specificities of polyclonal BARF0-specific CTL lines. CTL lines derived from PBMC from three seropositive donors (NK, LP, and AH) were tested after 17 days of culture in a standard 51Cr release assay against HLA A2-matched PHA blasts (from donor LL) which were either untreated or coated with BARF0 peptide 130 (PPRARDRA
LLWAARPRL
LLS) or 131 (WAARP
RLLLSLQQV
PEPRLA). Data from one representative experiment of three is presented. An E/T ratio of approximately 10:1 was used in the assay. The effectors were designated according to their donor origin and sensitizing peptide, e.g., NK-130 CTL indicates BARF0-specific CTLs from donor NK stimulated by peptide 130.
FIG. 3
Titration analysis for the minimal BARF0 epitope. 51Cr-labelled HLA A2-positive PHA blasts from donor NK were coated with different concentrations of overlapping 9-, 10-, 11-, or 12-aa-long peptide (all of which had the LLWAARPRL sequence) and assayed for recognition by the autologous polyclonal NK-130 CTL line (E/T ratio, approximately 15:1). Representative results from one of two experiments are shown. Conc., Concentration.
FIG. 4
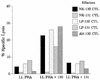
EBV-seropositive but not -seronegative individuals display a strong BARF0-specific CTL response. Polyclonal CTL lines (effectors) derived from PBMC from HLA A2, EBV-seropositive (EBV+) donors NK and JG or from seronegative (EBV−) donors KA and RM were tested in a standard 51Cr release assay against HLA A2 PHA blasts as targets. For effector designations, see the legend to Fig. 2. An E/T ratio of either 16:1 (for NK-130/CTL and KA-130/CTL) or 13:1 (for NK-LLW/CTL, KA-LLW/CTL, JG-LLW/CTL, and RM-LLW/CTL) was used. (A) The PHA blasts (from donors NK and KA) were either untreated or coated with BARF0 peptide 130 (PPRARDRA
LLWAARPRL
LLS) or LLWAARPRL (LLW). For negative controls, the HLA A2-restricted peptide YLQQNWWTL (YLQ) from LMP-1 (13) was used. The effector cells were tested after 17 days of culture. (B) PHA blasts from donor RM were either untreated or coated with BARF0 peptide LLWAARPRL (LLW). The effector cells were tested after 18 days of culture.
FIG. 5
EBV-positive BL cells and LCLs poorly present the BARF0 epitope. LCLs were generated by infection of B lymphocytes with EBV isolate B95.8 (LL LCL and SBLCL) or QIMR-Wil (NK LCL and DM LCL). The BL BL30 and the B-cell lymphoma BJAB are EBV-negative cell lines, whereas MutuI BL and Eli BL are EBV-positive BLs expressing viral latency I genes. MutuIII is an EBV-positive BL demonstrating a latency type III pattern. All of these cells were used as targets which were either untreated or incubated with peptide LLWAARPRL and analyzed in a standard 51Cr release assay against the polyclonal CTL line NK-130 (E/T ration, 10:1). Data from one representative experiment of three is shown. The HLA match between targets and effectors is indicated. NT, not tested.
FIG. 6
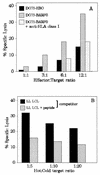
BARF0-transfected BL cells present the LLWAARPRL epitope. (A) The BL cell line DG75 (EBV negative, HLA A2 positive) was stably transfected with either a control vector (DG75-EBO) or a BARF0 expression vector (DG75-BARF0). These targets were analyzed in a standard 51Cr release assay with the CTL line NK-130 at different E/T ratios. DG75-BARF0 cells were additionally preincubated with an anti-HLA class I-specific antibody before the addition of effector cells. (B) 51Cr-labelled DG75-BARF0 cells were subjected to a cold target inhibition assay with either untreated or peptide 130-coated LL LCLs (competitor) at different hot/cold target ratios. CTL lysis was induced by the effector NK-130 CTL bulk culture at an E/T ratio of 10:1.
FIG. 7
RT-PCR of BARF0 expression. (A) Total RNA was reverse transcribed from three EBV-positive LCLs (LL, NK, and SB), two BL cell lines of type I viral latency (Eli and MutuI), a BL cell line of type III viral latency (MutuIII), the EBV-negative DG75 cells expressing the BARF0 gene (DG75-BARF0), or the vector control (DG75-EBO). For RT-negative controls, the RT enzyme was omitted (cont.−). Both a 131-bp fragment of β2-M and a 227-bp fragment of BARF0 were PCR amplified from the first-strand cDNAs and the BARF0 gene DNA (cont.+). These were separated and visualized by electrophoresis on an ethidium bromide-containing agarose gel. Data from one representative experiment of three is shown. (B) The RT-PCR fragments obtained from each of the cell lines were quantified, and the BARF0 signals were standardized against β2-M.
Similar articles
- Restoration of endogenous antigen processing in Burkitt's lymphoma cells by Epstein-Barr virus latent membrane protein-1: coordinate up-regulation of peptide transporters and HLA-class I antigen expression.
Rowe M, Khanna R, Jacob CA, Argaet V, Kelly A, Powis S, Belich M, Croom-Carter D, Lee S, Burrows SR, et al. Rowe M, et al. Eur J Immunol. 1995 May;25(5):1374-84. doi: 10.1002/eji.1830250536. Eur J Immunol. 1995. PMID: 7774641 - Characterization of an human leucocyte antigen A2-restricted Epstein-Barr virus nuclear antigen-1-derived cytotoxic T-lymphocyte epitope.
Marescotti D, Destro F, Baldisserotto A, Marastoni M, Coppotelli G, Masucci M, Gavioli R. Marescotti D, et al. Immunology. 2010 Mar;129(3):386-95. doi: 10.1111/j.1365-2567.2009.03190.x. Epub 2009 Nov 16. Immunology. 2010. PMID: 19922423 Free PMC article. - T cell recognition of Epstein-Barr virus associated lymphomas.
Rickinson AB, Murray RJ, Brooks J, Griffin H, Moss DJ, Masucci MG. Rickinson AB, et al. Cancer Surv. 1992;13:53-80. Cancer Surv. 1992. PMID: 1330300 Review. - Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection.
Rickinson AB, Moss DJ. Rickinson AB, et al. Annu Rev Immunol. 1997;15:405-31. doi: 10.1146/annurev.immunol.15.1.405. Annu Rev Immunol. 1997. PMID: 9143694 Review.
Cited by
- Increased frequency of antigen-specific CD8(+) cytotoxic T lymphocytes infiltrating an Epstein-Barr virus-associated gastric carcinoma.
Kuzushima K, Nakamura S, Nakamura T, Yamamura Y, Yokoyama N, Fujita M, Kiyono T, Tsurumi T. Kuzushima K, et al. J Clin Invest. 1999 Jul;104(2):163-71. doi: 10.1172/JCI6062. J Clin Invest. 1999. PMID: 10411545 Free PMC article. - Proteasomal inhibition triggers viral oncoprotein degradation via autophagy-lysosomal pathway.
Gain C, Malik S, Bhattacharjee S, Ghosh A, Robertson ES, Das BB, Saha A. Gain C, et al. PLoS Pathog. 2020 Feb 24;16(2):e1008105. doi: 10.1371/journal.ppat.1008105. eCollection 2020 Feb. PLoS Pathog. 2020. PMID: 32092124 Free PMC article. - Epstein-Barr virus BART microRNAs are produced from a large intron prior to splicing.
Edwards RH, Marquitz AR, Raab-Traub N. Edwards RH, et al. J Virol. 2008 Sep;82(18):9094-106. doi: 10.1128/JVI.00785-08. Epub 2008 Jul 9. J Virol. 2008. PMID: 18614630 Free PMC article. - Regulation of expression of the Epstein-Barr virus BamHI-A rightward transcripts.
Chen H, Huang J, Wu FY, Liao G, Hutt-Fletcher L, Hayward SD. Chen H, et al. J Virol. 2005 Feb;79(3):1724-33. doi: 10.1128/JVI.79.3.1724-1733.2005. J Virol. 2005. PMID: 15650197 Free PMC article.
References
- Baer R, Bankier A T, Biggin M D, Deininger P L, Farrell P J, Gibson T J, Hatfull G, Hudson G S, Satchwell S C, Seguin C, Tuffnell P S, Barrell B G. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984;310:207–211. - PubMed
- Ben Bassat H, Goldblum N, Mitrani S, Goldblum T, Yoffey J M, Cohen M M, Bentwich Z, Ramot B, Klein E, Klein G. Establishment in continuous culture of a new type of lymphocyte from a “Burkitt like” malignant lymphoma (line D.G.-75) Int J Cancer. 1977;19:27–33. - PubMed
- Central Data Analysis Committee. The data book of the 11th International Histocompatibility Workshop. Oxford, United Kingdom: Oxford University Press; 1991. pp. 807–814.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials