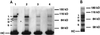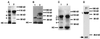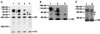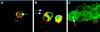Complex proteolytic processing acts on Delta, a transmembrane ligand for Notch, during Drosophila development - PubMed (original) (raw)
Complex proteolytic processing acts on Delta, a transmembrane ligand for Notch, during Drosophila development
K M Klueg et al. Mol Biol Cell. 1998 Jul.
Free PMC article
Abstract
Delta functions as a cell nonautonomous membrane-bound ligand that binds to Notch, a cell-autonomous receptor, during cell fate specification. Interaction between Delta and Notch leads to signal transduction and elicitation of cellular responses. During our investigations to further understand the biochemical mechanism by which Delta signaling is regulated, we have identified four Delta isoforms in Drosophila embryonic and larval extracts. We have demonstrated that at least one of the smaller isoforms, Delta S, results from proteolysis. Using antibodies to the Delta extracellular and intracellular domains in colocalization experiments, we have found that at least three Delta isoforms exist in vivo, providing the first evidence that multiple forms of Delta exist during development. Finally, we demonstrate that Delta is a transmembrane ligand that can be taken up by Notch-expressing Drosophila cultured cells. Cell culture experiments imply that full-length Delta is taken up by Notch-expressing cells. We present evidence that suggests this uptake occurs by a nonphagocytic mechanism.
Figures
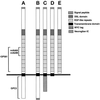
Figure 1
Schematic representation of Delta variants expressed in Drosophila cultured cells and the domains recognized by various Delta antibodies. (A) DeltaWT, full-length Delta. (B) DeltaWTNdeMYC, full-length Delta. (C) DeltaNGIC, Delta extracellular and transmembrane domains fused to a neuroglian intracellular domain. (D) DeltaDde, Delta protein truncated near the inner face of the plasma membrane. (E) DeltaSEC1, secretable Delta extracellular domain.
Figure 2
Immunoprecipitation of Delta protein from detergent-soluble native extracts of staged embryos and larvae using an antibody against the Delta extracellular domain (MAb8A), detected with Delta MAb9B. (A) Staged embryonic extracts (6 mg wet weight/ml) immunoprecipitated using a mAb against the Delta extracellular domain (MAb8A). The same volume of extract was used for immunoprecipitation for each time point. Ages of pooled animals, in hours postoviposition (PO) at 25°C, for each lane are: lane 1, 0–6 h; lane 2, 7–12 h; lane 3, 13–18 h; lane 4, 19–24 h. The bracket next to “I1” indicates that more than one band is detected in the 92- to 96-kDa range. “HC” indicates the IgG heavy chain from the mouse ascites used in the immunoprecipitation. (B) Immunoprecipitation of Delta protein from third instar larval extracts (180 mg wet weight/ml) using MAb8A.
Figure 3
Identification of Delta isoforms in cultured cells. (A and B) Immunoprecipitation of Delta protein from detergent-soluble native extracts of staged embryos and cultured_Drosophila_ S2 cells using an antibody against the Delta extracellular domain (MAb8A), detected with MAb9B. (A) Comparison of Delta isoforms from embryos 7–12 h PO (lane 1) with Delta isoforms from cultured cells programmed to express full-length Delta protein (∼98 kDa, lane 2). (B) Comparison of Delta isoforms from cultured cells programmed to express a secreted form of the Delta extracellular domain (∼65 kDa, lane 1) with Delta isoforms from an extract of embryos 0–22 h PO (lane 2). (C) Comparison of Delta isoforms immunoprecipitated using MAb8A from cultured cells that express DeltaWTNdeMYC (lane 1) with Delta isoforms immunoprecipitated from DeltaWTNdeMYC+ cells with MAb9E, which binds to the MYC epitope at the C terminus of the intracellular domain (lane 2). (D) Total protein sample from DeltaWTNdeMYC+ cells, probed with MAb9E. Arrow indicates a protein species (∼22 kDa) that reacts with an antibody to the MYC tag at the C terminus of the Delta intracellular domain.
Figure 4
Immunoprecipitation of Delta protein from detergent-soluble native extracts of staged embryos and larvae using MAb8A against the Delta extracellular domain, detected with MAb9B. (A) Immunoprecipitation of Delta from staged embryonic extracts (300 mg wet weight/ml). The same volume of extract was used for immunoprecipitation for each time point. Ages of pooled animals, in hours PO at 25°C, for each lane were: lane 1, 0–6 h; lane 2, 7–12 h; lane 3, 13–18 h; lane 4, 19–24 h. (B) Immunoprecipitation of Delta from mixed-stage embryonic extracts. Extracts from embryos 22–24 h PO mixed with embryos 2.5–4.5 h PO before extraction and immunoprecipitation are compared with extracts from the single samples (taken from the same egg collections used to prepare the mixed samples): lane 1, 2.5–4.5 h (110 mg/ml); lane 2, 2.5–4.5 h plus 22–24 h (220 mg/ml); lane 3, 22–24 h (110 mg/ml). (C) Immunoprecipitation of Delta from extracts of a mixture of embryos 3–5 h PO and second instar larvae compared with an extract made from embryos 3–5 h PO: lane 1, 3–5 h (45 mg/ml); lane 2, 3–5 h mixed with second instar larvae (a total of 120 mg/ml, of which 45 mg/ml was embryonic tissue 3–5 h PO and 72 mg/ml was second instar larval tissue). The contribution of Delta isoforms from second instar larvae is negligible, based on previous experiments in which it was determined that a minimum of 180 mg/ml of larvae are needed to detect larval Delta isoforms by immunoprecipitation (our unpublished results).
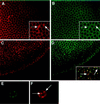
Figure 5
Immunofluorescent localization of Delta extracellular and intracellular domains in cellular blastoderm embryos, and in cultured cells programmed to express DeltaWTNdeMYC. (A) Cellular blastoderm stained with MAb9B to the Delta extracellular domain. (B) Same embryo as in panel A, showing localization of the Delta intracellular domain (C2 guinea pig polyclonal antisera). Insets in panels A and B, a section of the larger panel presented at higher magnification, illustrate the difference between the localization of Delta extracellular and intracellular domains (arrows). (C) Cellular blastoderm embryo, immediately before gastrulation, showing localization of the Delta extracellular domain detected with MAb9B. At this stage, Delta is plasma membrane-associated, except in the mesodermal anlage where Delta accumulates in vesicles. (D) Same embryo as in panel C, showing localization of the Delta intracellular domain with C2 guinea pig polyclonal antisera. Inset in panel D shows a high magnification, merged image of the same area of panels C and D, to illustrate that Delta extracellular and intracellular domains colocalize in vesicular structures. However, some vesicular structures react with only one of the two domain-specific antibodies (arrows). (E and F) A cultured cell programmed to express DeltaWTNdeMYC. The Delta extracellular domain is detected with GP581 guinea pig polyclonal antiserum in panel E, and localization of the intracellular domain is assessed with MAb9E in panel F. Arrows in panel F indicate vesicles that reacts only with MAb9E.
Figure 6
_Trans-_uptake of Delta and Notch in Drosophila S2 cell aggregation assays. In panels A-C, antibodies to Delta are detected with fluorescein-conjugated secondary antibodies (green); antibodies to Notch are detected with Texas Red-conjugated secondary antibodies (red). These panels present merged confocal images. Colocalized Delta and Notch appear yellow. (A) Antibodies to the Delta extracellular domain colocalize with antibodies to the Notch intracellular domain in vesicular structures within a Notch+ cell (arrow). (B) Antibodies to the Delta intracellular domain colocalize with antibodies to the Notch intracellular domain in vesicular structures within a Notch+ cell (arrow). (C) Notch+ cells aggregated with cells that express DeltaNGIC, a Delta-neuroglian chimera, in which the Delta intracellular domain is replaced with the neuroglian intracellular domain. Antibodies to the Delta extracellular domain colocalize with antibodies to the Notch intracellular domain within vesicles in Notch+ cells (arrows). (D) In Notch+ cells aggregated with cells that express DeltaNGIC, antibodies to the neuroglian intracellular domain (green) colocalize with antibodies to the Notch extracellular domain (red) within vesicles in Notch+ cells (arrows). (E) In Notch+ cells aggregated with cells that express DeltaDde, which lacks the Delta intracellular domain, antibodies to the Delta extracellular domain (green) colocalize with antibodies to the Notch intracellular domain (red), within vesicles in Notch+ cells (arrows). (F and G) Delta–Notch aggregates stained with antibodies to the Delta extracellular domain (green) and antibodies to the Delta intracellular domain (red). This merged image shows Notch+ cells that contain vesicles (which appear yellow) that costain with the Delta extracellular and intracellular domain-specific antibodies. Some vesicles that are recognized by only the extracellular domain antibody (MAb9B, green vesicles, arrow labeled “ec”) or by only the intracellular domain antibody (C2 guinea pig polyclonal antiserum, red vesicles, arrows labeled “ic”) are also found. (H) In cells that express full-length Delta aggregated with cells that express full-length Notch, antibodies to the Notch intracellular domain (red) colocalize with antibodies to the Delta extracellular domain (green) in Delta+ cells (arrows). The asterisk indicates a Delta+ vesicle inside a Notch+ cell.
Figure 7
Localization of plasma membrane proteins during intercellular transfer. (A) Delta–Notch aggregates in which boss is coexpressed on the cell surface with Delta. Aggregates are stained with antibodies to the Delta extracellular domain (GP581, green) and with antibodies to boss (mAb-αboss1, red). The asterisk indicates a Delta+ vesicle in an adjacent Notch+ cell; this vesicle is not boss+. Vesicular structures within Delta+ cells stain with Delta and boss antibodies (arrow). (B) Delta-Notch aggregates in which neuroglian (red) is expressed on the cell surface of Notch+ cells (green). Arrows indicate Notch+ vesicles in Delta+ cells; these vesicles are not neuroglian+. (C) Delta–Notch aggregates in which neuroglian (green) is expressed on the surfaces of Delta+ cells. Cells were also labeled for Notch (red). Arrow indicates a neuroglian+/Notch+ vesicle in a Delta+ cell.
Similar articles
- The Notch ligands, Jagged and Delta, are sequentially processed by alpha-secretase and presenilin/gamma-secretase and release signaling fragments.
LaVoie MJ, Selkoe DJ. LaVoie MJ, et al. J Biol Chem. 2003 Sep 5;278(36):34427-37. doi: 10.1074/jbc.M302659200. Epub 2003 Jun 25. J Biol Chem. 2003. PMID: 12826675 - Notch-induced proteolysis and nuclear localization of the Delta ligand.
Bland CE, Kimberly P, Rand MD. Bland CE, et al. J Biol Chem. 2003 Apr 18;278(16):13607-10. doi: 10.1074/jbc.C300016200. Epub 2003 Feb 18. J Biol Chem. 2003. PMID: 12591935 - Processing of the notch ligand delta by the metalloprotease Kuzbanian.
Qi H, Rand MD, Wu X, Sestan N, Wang W, Rakic P, Xu T, Artavanis-Tsakonas S. Qi H, et al. Science. 1999 Jan 1;283(5398):91-4. doi: 10.1126/science.283.5398.91. Science. 1999. PMID: 9872749 - [Locus Delta in the Notch signaling system: organization and pleiotropic function during Drosophila development].
Gubenko IS. Gubenko IS. Tsitol Genet. 2001 Jul-Aug;35(4):59-80. Tsitol Genet. 2001. PMID: 11833341 Review. Russian. - Notch signaling: a dance of proteins changing partners.
Kadesch T. Kadesch T. Exp Cell Res. 2000 Oct 10;260(1):1-8. doi: 10.1006/excr.2000.4921. Exp Cell Res. 2000. PMID: 11010805 Review. No abstract available.
Cited by
- Drosophila mauve mutants reveal a role of LYST homologs late in the maturation of phagosomes and autophagosomes.
Rahman M, Haberman A, Tracy C, Ray S, Krämer H. Rahman M, et al. Traffic. 2012 Dec;13(12):1680-92. doi: 10.1111/tra.12005. Epub 2012 Sep 20. Traffic. 2012. PMID: 22934826 Free PMC article. - The Membrane-Bound Notch Regulator Mnr Supports Notch Cleavage and Signaling Activity in Drosophila melanogaster.
Nagel AC, Müller D, Zimmermann M, Preiss A. Nagel AC, et al. Biomolecules. 2021 Nov 10;11(11):1672. doi: 10.3390/biom11111672. Biomolecules. 2021. PMID: 34827670 Free PMC article. - Down-regulation of Delta by proteolytic processing.
Mishra-Gorur K, Rand MD, Perez-Villamil B, Artavanis-Tsakonas S. Mishra-Gorur K, et al. J Cell Biol. 2002 Oct 28;159(2):313-24. doi: 10.1083/jcb.200203117. Epub 2002 Oct 28. J Cell Biol. 2002. PMID: 12403816 Free PMC article. - Endocytosis and intracellular trafficking of Notch and its ligands.
Yamamoto S, Charng WL, Bellen HJ. Yamamoto S, et al. Curr Top Dev Biol. 2010;92:165-200. doi: 10.1016/S0070-2153(10)92005-X. Curr Top Dev Biol. 2010. PMID: 20816395 Free PMC article. Review. - Connexin 43 is an emerging therapeutic target in ischemia/reperfusion injury, cardioprotection and neuroprotection.
Schulz R, Görge PM, Görbe A, Ferdinandy P, Lampe PD, Leybaert L. Schulz R, et al. Pharmacol Ther. 2015 Sep;153:90-106. doi: 10.1016/j.pharmthera.2015.06.005. Epub 2015 Jun 11. Pharmacol Ther. 2015. PMID: 26073311 Free PMC article. Review.
References
- Artavanis-Tsakonas S, Matsuno K, Fortini ME. Notch signaling. Science. 1995;268:225–232. - PubMed
- Authier F, Posner BI, Bergeron JJM. Endosomal proteolysis of internalized proteins. FEBS Lett. 1996;389:55–60. - PubMed
- Baker R, Schubiger G. Autonomous and nonautonomous Notch functions for embryonic muscle and epidermis development in Drosophila. Development. 1996;122:617–626. - PubMed
- Bate, M., Rushton, E., and Frasch, M. (1993). A dual requirement for neurogenic genes in Drosophila myogenesis. Dev. Suppl. 149–161. - PubMed
- Bieber AJ, Snow PM, Hortsch M, Patel NH, Jacobs JR, Traquina ZR, Schilling J, Goodman CS. Drosophila neuroglian: a member of the immunogobulin superfamily with extensive homology to the vertebrate neural adhesion molecule L1. Cell. 1989;59:447–460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous