The yeast dynactin complex is involved in partitioning the mitotic spindle between mother and daughter cells during anaphase B - PubMed (original) (raw)
The yeast dynactin complex is involved in partitioning the mitotic spindle between mother and daughter cells during anaphase B
J A Kahana et al. Mol Biol Cell. 1998 Jul.
Free PMC article
Abstract
Although vertebrate cytoplasmic dynein can move to the minus ends of microtubules in vitro, its ability to translocate purified vesicles on microtubules depends on the presence of an accessory complex known as dynactin. We have cloned and characterized a novel gene, NIP100, which encodes the yeast homologue of the vertebrate dynactin complex protein p150(glued). Like strains lacking the cytoplasmic dynein heavy chain Dyn1p or the centractin homologue Act5p, nip100Delta strains are viable but undergo a significant number of failed mitoses in which the mitotic spindle does not properly partition into the daughter cell. Analysis of spindle dynamics by time-lapse digital microscopy indicates that the precise role of Nip100p during anaphase is to promote the translocation of the partially elongated mitotic spindle through the bud neck. Consistent with the presence of a true dynactin complex in yeast, Nip100p exists in a stable complex with Act5p as well as Jnm1p, another protein required for proper spindle partitioning during anaphase. Moreover, genetic depletion experiments indicate that the binding of Nip100p to Act5p is dependent on the presence of Jnm1p. Finally, we find that a fusion of Nip100p to the green fluorescent protein localizes to the spindle poles throughout the cell cycle. Taken together, these results suggest that the yeast dynactin complex and cytoplasmic dynein together define a physiological pathway that is responsible for spindle translocation late in anaphase.
Figures
Figure 1
Similarity between Nip100p and p150_glued_. (A) Sequence alignment of the N-terminal regions of Nip100p and p150_glued_. Identical residues are shown in inverse; similar residues are boxed. Sequence alignment was performed using the Megalign module from the Lasergene software package (DNAstar, Madison, WI). (B) Predicted coiled-coil structure of Nip100p and rat p150_glued_. Predictions were performed using the COILS algorithm of Lupas et al. (1991) with a window of 28 residues.
Figure 2
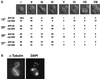
Anaphase defect of _nip100_Δ cells. (A) Spindle morphologies of wild-type and nip100_Δ strains. Strains JKY85 (wild-type) and JKY86 (nip100_Δ::HIS3) expressing the Nuf2-GFP SPB tag were grown to OD600 = 0.6–0.8 and observed on a fluorescence microscope with an FITC filter set. An overlay of Nuf2-GFP with a differential interference contrast image of cells representing each of the typical morphologies is shown along with the percentage of wild-type and _nip100_Δ cells exhibiting each morphology at 12, 25, 30, and 37°C (n > 100 cells for each sample). Morphologies I–IV are wild-type (G1, S, G2–M, and M phase, respectively), whereas morphologies V–VIII represent the mutant phenotype of_nip100_Δ cells. (B) Fluorescence micrographs of a typical type V _nip100_Δ cell. Microtubules were visualized by indirect immunofluorescence using a monoclonal antibody against α-tubulin. DNA was visualized with DAPI. Note that, although the spindle has failed to enter the daughter cell, astral microtubules have passed through the bud neck, and sister chromatids have separated.
Figure 3
In vivo analysis of spindle movements in wild-type and _nip100_Δ cells. Wild-type JKY85 (A and B) and _nip100_Δ (JKY86) (C and D) cells expressing Nuf2-GFP were observed by time-lapse fluorescence microscopy. Live cells were observed at 10-s intervals for up to 25 min. Elapsed times are shown are in seconds. Arrows denote the position of the bud neck. (A and B) Rapid penetration of mitotic spindle through the bud neck in wild-type cells. Note that wild type cells typically translocate their mitotic spindles through the bud neck within 3 min of the advent of anaphase spindle elongation. (C) Mutant_nip100_Δ cells generally do not translocate their spindles through the bud neck. Note that the spindle elongates to ∼4 μm within the mother but does not translocate into the daughter. However at ∼270 s after anaphase B begins, continuing spindle elongation causes the daughter spindle pole to pass through the bud neck. (D) A mother–daughter pair of _nip100_Δ cells, which fails to partition the spindle into the bud for an extended period. Note that, like the cells in C, the spindle has elongated to the mother cell cortex. However, the spindle remains in the mother for >14 min. However, at 1340 s, the spindle has elongated through the bud neck.
Figure 4
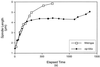
Kinetic analysis of spindle elongation in_nip100_Δ mutants. Kinetic diagrams of wild-type (open squares) and _nip100_Δ (filled diamonds) cells. The_nip100_Δ cell pair is shown in Figure 3D. Fast elongation for both cells (0–200 s; spindle length, <4 μm) occurs at 0.89 μm/min. Slow elongation (200–1500 s) occurs at ∼0.20 μm/min in both wild-type and _nip100_Δ mutant cells. However, elongation pauses for >10 min (∼300–1100 s) in the_nip100_Δ cell while the spindle is stuck within the mother cell. Rates were determined as the slope of a linear curve fit of the data points in each phase.
Figure 5
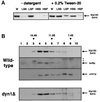
Biochemical characterization of the yeast dynactin complex. (A) Detergent solubility of Nip100-3xHA from whole-cell extracts. Cells expressing Nip100-3xHA were lysed in buffer with or without 0.2% Tween 20 nonionic detergent. The lysate was spun at 13,000 rpm in a microcentrifuge, and the low-speed supernatant was then spun at 55,000 rpm (∼100,000 × g) in a tabletop ultracentrifuge. Equal fractions of the whole-cell extract (W), low-speed supernatant (LSS), low-speed pellet (LSP), high-speed supernatant (HSS), and high-speed pellet (HSP) were analyzed by immunoblotting with the 12CA5 anti-HA monoclonal antibody. (B) Sucrose density gradient centrifugation indicates that Nip100p, Act5p, and Jnm1p are all present in a 15.5S complex, which does not include cytoplasmic dynein. High-speed supernatants from wild-type or _dyn1_Δ strains expressing Nip100-3xHA were analyzed in 7–18% sucrose gradients. Ten equal fractions were collected and analyzed by SDS-PAGE and immunoblotting using antibodies against the HA tag (wild-type panel, top row; and dyn1Δ panel), Act5p (wild-type panel, second row), and Jnm1p (wild-type panel, third row). Cross-reacting bands detected by the antiAct5p antibody are denoted with an asterisk. Purified thyroglobulin (19.4S), catalase (11.3S), and yeast alcohol dehydrogenase (7.4S) were used as standards.
Figure 6
Direct biochemical interactions between dynactin homologues and dynein chains. Cells expressing HA-tagged Dyn1p (Dyn1-3xHA) and various protein A IgG binding site (4Z)–tagged proteins were lysed under nondenaturing conditions, and the extracts were incubated with human IgG-Sepharose beads. After washing, bound (B) and free (F) proteins were resolved by SDS-PAGE and blotted to nitrocellulose. Blots were probed with antibodies against Act5p, Jnm1p, and the HA tag. The bound fractions were concentrated 10-fold (with respect to free fractions) before electrophoresis. (A) Act5p and Jnm1p specifically coprecipitate with Nip100-4Z (lanes 1 and 2) but not with Nuf2-4Z (lanes 3 and 4), 4Z (lanes 5 and 6), or Pac11-4Z (lanes 7 and 8). Note that Act5p migrates at the expected 42 kDa, whereas Jnm1p (predicted molecular mass, 43.6 kDa) migrates at ∼50 kDa. The heavy chain of cytoplasmic dynein (Dyn1-3xHA) specifically coprecipitates only with the intermediate chain (Pac11-4Z). (B) Wild-type (lanes 1 and 2), _act5_Δ (lanes 3 and 4), and _jnm1_Δ (lanes 5 and 6) cells expressing Nip100-4Z were processed as in A. Although the absence of Act5p has no effect on Jnm1p binding to Nip100-4Z, there is a 5- to 10-fold decrease in binding of Act5p to Nip100-4Z in a _jnm1_Δ strain.
Figure 7
Nip100 localizes to the spindle poles of vegetatively growing cells throughout the cell cycle. (A) Strain JKY77 (nip100_Δ::HIS3_) overexpressing Nip100-GFP from a 2μ plasmid was grown to midlogarithmic phase and processed for immunofluorescence. Nip100-GFP was visualized with a polyclonal antibody against GFP followed by FITC-conjugated secondary antibodies. Microtubules were visualized with a monoclonal antibody against α-tubulin followed by Texas Red-conjugated secondary antibodies. Chromatin was visualized with DAPI. (B) Pulse–chase expression of Nip100-GFP using the_GAL1–10_ promoter. Nip100-GFP was expressed for 30 min using 2% galactose and then repressed for 3 h using 2% glucose as the sole carbon source. In 58% of large-budded cells, Nip100-GFP is predominantly localized to the SPB, which is in the daughter cell (top two panels). In small and medium (preanaphase B) cells, Nip100-GFP is typically associated with a single SPB at the bud neck (bottom panel).
Similar articles
- Dynein-driven mitotic spindle positioning restricted to anaphase by She1p inhibition of dynactin recruitment.
Woodruff JB, Drubin DG, Barnes G. Woodruff JB, et al. Mol Biol Cell. 2009 Jul;20(13):3003-11. doi: 10.1091/mbc.e09-03-0186. Epub 2009 Apr 29. Mol Biol Cell. 2009. PMID: 19403691 Free PMC article. - A yeast actin-related protein homologous to that in vertebrate dynactin complex is important for spindle orientation and nuclear migration.
Muhua L, Karpova TS, Cooper JA. Muhua L, et al. Cell. 1994 Aug 26;78(4):669-79. doi: 10.1016/0092-8674(94)90531-2. Cell. 1994. PMID: 8069915 - Dynactin targets Pavarotti-KLP to the central spindle during anaphase and facilitates cytokinesis in Drosophila S2 cells.
Delcros JG, Prigent C, Giet R. Delcros JG, et al. J Cell Sci. 2006 Nov 1;119(Pt 21):4431-41. doi: 10.1242/jcs.03204. Epub 2006 Oct 17. J Cell Sci. 2006. PMID: 17046997 - Role of NuMA in vertebrate cells: review of an intriguing multifunctional protein.
Sun QY, Schatten H. Sun QY, et al. Front Biosci. 2006 Jan 1;11:1137-46. doi: 10.2741/1868. Front Biosci. 2006. PMID: 16146802 Review. - Mitotic motors in Saccharomyces cerevisiae.
Hildebrandt ER, Hoyt MA. Hildebrandt ER, et al. Biochim Biophys Acta. 2000 Mar 17;1496(1):99-116. doi: 10.1016/s0167-4889(00)00012-4. Biochim Biophys Acta. 2000. PMID: 10722880 Review.
Cited by
- Mitotic spindle form and function.
Winey M, Bloom K. Winey M, et al. Genetics. 2012 Apr;190(4):1197-224. doi: 10.1534/genetics.111.128710. Genetics. 2012. PMID: 22491889 Free PMC article. - The cortical protein Num1p is essential for dynein-dependent interactions of microtubules with the cortex.
Heil-Chapdelaine RA, Oberle JR, Cooper JA. Heil-Chapdelaine RA, et al. J Cell Biol. 2000 Dec 11;151(6):1337-44. doi: 10.1083/jcb.151.6.1337. J Cell Biol. 2000. PMID: 11121446 Free PMC article. - Prioritized Expression of BTN2 of Saccharomyces cerevisiae under Pronounced Translation Repression Induced by Severe Ethanol Stress.
Yamauchi Y, Izawa S. Yamauchi Y, et al. Front Microbiol. 2016 Aug 23;7:1319. doi: 10.3389/fmicb.2016.01319. eCollection 2016. Front Microbiol. 2016. PMID: 27602028 Free PMC article. - Bim1p/Yeb1p mediates the Kar9p-dependent cortical attachment of cytoplasmic microtubules.
Miller RK, Cheng SC, Rose MD. Miller RK, et al. Mol Biol Cell. 2000 Sep;11(9):2949-59. doi: 10.1091/mbc.11.9.2949. Mol Biol Cell. 2000. PMID: 10982392 Free PMC article. - Arp10p is a pointed-end-associated component of yeast dynactin.
Clark SW, Rose MD. Clark SW, et al. Mol Biol Cell. 2006 Feb;17(2):738-48. doi: 10.1091/mbc.e05-05-0449. Epub 2005 Nov 16. Mol Biol Cell. 2006. PMID: 16291862 Free PMC article.
References
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials