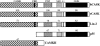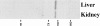Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells - PubMed (original) (raw)
Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells
A R Cohen et al. J Cell Biol. 1998.
Erratum in
- J Cell Biol 1998 Aug 24;142(4):following 1156. Wood DF [corrected to Woods DF]
Abstract
In Caenorhabditis elegans, mutations in the lin-2 gene inactivate the LET-23 receptor tyrosine kinase/Ras/MAP kinase pathway required for vulval cell differentiation. One function of LIN-2 is to localize LET-23 to the basal membrane domain of vulval precursor cells. LIN-2 belongs to the membrane-associated guanylate kinase family of proteins. We have cloned and characterized the human homolog of LIN-2, termed hCASK, and Northern and Western blot analyses reveal that it is ubiquitously expressed. Indirect immunofluorescence localizes CASK to distinct lateral and/or basal plasma membrane domains in different epithelial cell types. We detect in a yeast two-hybrid screen that the PDZ domain of hCASK binds to the heparan sulfate proteoglycan syndecan-2. This interaction is confirmed using in vitro binding assays and immunofluorescent colocalization. Furthermore, we demonstrate that hCASK binds the actin-binding protein 4.1. Syndecans are known to bind extracellular matrix, and to form coreceptor complexes with receptor tyrosine kinases. We speculate that CASK mediates a link between the extracellular matrix and the actin cytoskeleton via its interaction with syndecan and with protein 4.1. Like other membrane-associated guanylate kinases, its multidomain structure enables it to act as a scaffold at the membrane, potentially recruiting multiple proteins and coordinating signal transduction.
Figures
Figure 1
Comparative domain organization of hCASK-related proteins. Sequence homology of hCASK with rat CASK (Hata et al., 1996), C. elegans LIN-2 (Hoskins et al., 1995), human erythrocyte p55 (Ruff et al., 1991), and alpha subunit of rat brain CAMKII (Lin et al., 1987). hCASK protein contains a domain homologous to CAMKII, a conserved calmodulin-binding domain (CBD), a PDZ domain, an SH3 domain, a potential protein 4.1–binding motif (4.1), and a domain homologous to guanylate kinase (GUK). Percent amino acid identities within defined domains are noted in comparison with hCASK.
Figure 2
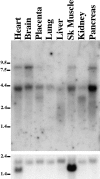
hCASK is ubiquitously expressed. Northern blot of human poly(A)+ mRNA probed with hCASK cDNA. The tissue source of the blotted RNA is indicated above each lane. Three differently sized transcripts are apparent at 8.5 kb, 4.4 kb, and 3.1 kb. All are large enough to encode full-length cDNA (2.765 kb). The position of size markers are indicated on the left (kb). Hybridization to 1.9-kb β-actin transcript is shown in the bottom panel as a loading control. Heart and skeletal muscle characteristically show a second β-actin mRNA form at 1.5 kb.
Figure 3
Anti-hCASK antibodies recognize one band at 112 kD, consistent with full-length cDNA and the nematode homolog. Immunoblot of rat tissues probed with affinity-purified anti-hCASK antibodies. Whole tissue samples were normalized by total protein, and were resolved by SDS-PAGE (10%). Positions of molecular weight markers are shown.
Figure 4
CASK localizes to distinct membrane domains in different epithelial cell types. Rat tissue sections were stained with affinity-purified anti-hCASK antibodies. (a) In choroid plexus epithelial cells, CASK localizes along the basal membrane; bar, 100 μm. (b) In colonic epithelial cells, CASK is enriched at the base of the lateral plasma membrane; bar, 40 μm.
Figure 6
CASK and syndecan-1 colocalize in situ in epithelial tissues. Mouse tissue sections were stained with affinity-purified rabbit anti-hCASK and monoclonal rat anti-syndecan-1 antibodies and visualized with Texas red–conjugated anti-rabbit and FITC-conjugated anti-rat secondary antibodies, respectively. (a, b) In hepatocytes, both CASK and syndecan-1 are located along the basal sinusoidal membrane. (c, d) In small intestinal enterocytes, the two proteins are localized at the lateral plasma membrane. CASK also stains a subset of the vessels that syndecan-1 stains in the lamina propria. (e, f) In the pancreas, CASK and syndecan-1 colocalize on both the basal and lateral membrane domains of acinar cells; bar, 40 μm.
Figure 5
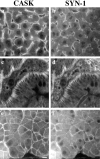
The PDZ domain of hCASK binds to the COOH-terminal sequence of syndecans. (a) Alignment of the cytoplasmic domains of rat syndecan family members demonstrate the high degree of homology among the four proteins (reviewed in Bernfield et al., 1992). In particular, note the conservation of the last four residues (in bold), which in other proteins are known to determine PDZ tail-binding specificity. (b) Direct binding study with syn-2 peptide and hCASK PDZ fusion protein. Syn-2 peptide representing the last eight residues was coupled to CNBr- activated Sepharose beads. The beads with immobilized peptide were incubated with GST-PDZ recombinant fusion protein with increasing concentrations of syn-2 or syn-1 peptides (0–200 μM) in solution. Controls consisted of GST alone incubated with peptides immobilized on beads; GST-PDZ fusion protein with peptide-free Sepharose beads (Beads alone); and GST-PDZ fusion protein incubated with beads coupled to syn-2 peptide and 50 μM control dlg peptide in solution (Ctr). The dlg control peptide (KKKKETDV-COOH) has optimal binding affinity for murine Dlg PDZ domain (Songyang et al., 1997).
Figure 5
The PDZ domain of hCASK binds to the COOH-terminal sequence of syndecans. (a) Alignment of the cytoplasmic domains of rat syndecan family members demonstrate the high degree of homology among the four proteins (reviewed in Bernfield et al., 1992). In particular, note the conservation of the last four residues (in bold), which in other proteins are known to determine PDZ tail-binding specificity. (b) Direct binding study with syn-2 peptide and hCASK PDZ fusion protein. Syn-2 peptide representing the last eight residues was coupled to CNBr- activated Sepharose beads. The beads with immobilized peptide were incubated with GST-PDZ recombinant fusion protein with increasing concentrations of syn-2 or syn-1 peptides (0–200 μM) in solution. Controls consisted of GST alone incubated with peptides immobilized on beads; GST-PDZ fusion protein with peptide-free Sepharose beads (Beads alone); and GST-PDZ fusion protein incubated with beads coupled to syn-2 peptide and 50 μM control dlg peptide in solution (Ctr). The dlg control peptide (KKKKETDV-COOH) has optimal binding affinity for murine Dlg PDZ domain (Songyang et al., 1997).
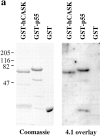
Figure 7
hCASK binds to the actin/spectrin-binding protein 4.1. (a) [125I]-labeled 30-kD fragment of protein 4.1 was incubated with GST-hCASK, GST-p55, and GST alone immobilized on nitrocellulose. Radiolabeled protein 4.1 bound GST-hCASK and GST-p55, but not GST alone. (Left) SDS-PAGE of expressed fusion proteins used for the experiment. (b)125I-labeled 30-kD fragment of protein 4.1 was incubated with GST-hCASK and GST alone coupled to glutathione Sepharose beads. Significantly more radiolabeled protein 4.1 bound to GST-hCASK than to GST alone, and the binding was effectively competed by incubation with an excess of nonradiolabeled protein 4.1. Experiments were performed in triplicate.
Figure 7
hCASK binds to the actin/spectrin-binding protein 4.1. (a) [125I]-labeled 30-kD fragment of protein 4.1 was incubated with GST-hCASK, GST-p55, and GST alone immobilized on nitrocellulose. Radiolabeled protein 4.1 bound GST-hCASK and GST-p55, but not GST alone. (Left) SDS-PAGE of expressed fusion proteins used for the experiment. (b)125I-labeled 30-kD fragment of protein 4.1 was incubated with GST-hCASK and GST alone coupled to glutathione Sepharose beads. Significantly more radiolabeled protein 4.1 bound to GST-hCASK than to GST alone, and the binding was effectively competed by incubation with an excess of nonradiolabeled protein 4.1. Experiments were performed in triplicate.
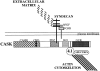
Figure 8
A hypothetical model of hCASK mediating a direct link between extracellular matrix and actin cytoskeleton by binding to syndecan and protein 4.1. hCASK binds to syndecan via its PDZ domain, and to protein 4.1 presumably through a sequence motif located between the SH3 and the GUK domains, as previously demonstrated for p55 and hDlg. Syndecans have been shown to bind extracellular matrix components and to form noncovalent coreceptor complexes with receptor tyrosine kinases, such as bFGFR. hCASK, in close proximity to the receptor tyrosine kinase, could act on the cytoplasmic surface to recruit proteins used in signaling events downstream of the bFGF receptor, or alternatively, could itself be a substrate for the receptor.
Similar articles
- Direct interaction of CASK/LIN-2 and syndecan heparan sulfate proteoglycan and their overlapping distribution in neuronal synapses.
Hsueh YP, Yang FC, Kharazia V, Naisbitt S, Cohen AR, Weinberg RJ, Sheng M. Hsueh YP, et al. J Cell Biol. 1998 Jul 13;142(1):139-51. doi: 10.1083/jcb.142.1.139. J Cell Biol. 1998. PMID: 9660869 Free PMC article. - Bipartite interaction between neurofibromatosis type I protein (neurofibromin) and syndecan transmembrane heparan sulfate proteoglycans.
Hsueh YP, Roberts AM, Volta M, Sheng M, Roberts RG. Hsueh YP, et al. J Neurosci. 2001 Jun 1;21(11):3764-70. doi: 10.1523/JNEUROSCI.21-11-03764.2001. J Neurosci. 2001. PMID: 11356864 Free PMC article. - CASK participates in alternative tripartite complexes in which Mint 1 competes for binding with caskin 1, a novel CASK-binding protein.
Tabuchi K, Biederer T, Butz S, Sudhof TC. Tabuchi K, et al. J Neurosci. 2002 Jun 1;22(11):4264-73. doi: 10.1523/JNEUROSCI.22-11-04264.2002. J Neurosci. 2002. PMID: 12040031 Free PMC article. - Syndecan, a developmentally regulated cell surface proteoglycan that binds extracellular matrix and growth factors.
Bernfield M, Sanderson RD. Bernfield M, et al. Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):171-86. doi: 10.1098/rstb.1990.0052. Philos Trans R Soc Lond B Biol Sci. 1990. PMID: 1969657 Review. - Genetic studies define MAGUK proteins as regulators of epithelial cell polarity.
Caruana G. Caruana G. Int J Dev Biol. 2002;46(4):511-8. Int J Dev Biol. 2002. PMID: 12141438 Review.
Cited by
- Scaffolding proteins DLG1 and CASK cooperate to maintain the nephron progenitor population during kidney development.
Ahn SY, Kim Y, Kim ST, Swat W, Miner JH. Ahn SY, et al. J Am Soc Nephrol. 2013 Jun;24(7):1127-38. doi: 10.1681/ASN.2012111074. Epub 2013 May 9. J Am Soc Nephrol. 2013. PMID: 23661808 Free PMC article. - Regulation of potassium channel trafficking in the distal nephron.
Welling PA. Welling PA. Curr Opin Nephrol Hypertens. 2013 Sep;22(5):559-65. doi: 10.1097/MNH.0b013e328363ff76. Curr Opin Nephrol Hypertens. 2013. PMID: 23892700 Free PMC article. Review. - TIP-1 has PDZ scaffold antagonist activity.
Alewine C, Olsen O, Wade JB, Welling PA. Alewine C, et al. Mol Biol Cell. 2006 Oct;17(10):4200-11. doi: 10.1091/mbc.e06-02-0129. Epub 2006 Jul 19. Mol Biol Cell. 2006. PMID: 16855024 Free PMC article. - Neuronal cell adhesion genes: Key players in risk for schizophrenia, bipolar disorder and other neurodevelopmental brain disorders?
Corvin AP. Corvin AP. Cell Adh Migr. 2010 Oct-Dec;4(4):511-4. doi: 10.4161/cam.4.4.12460. Cell Adh Migr. 2010. PMID: 20574149 Free PMC article. - Functions of Rhotekin, an Effector of Rho GTPase, and Its Binding Partners in Mammals.
Ito H, Morishita R, Nagata KI. Ito H, et al. Int J Mol Sci. 2018 Jul 20;19(7):2121. doi: 10.3390/ijms19072121. Int J Mol Sci. 2018. PMID: 30037057 Free PMC article. Review.
References
- Aroia RV, Koga M, Mendel JE, Ohshima Y, Sternberg PW. The let-23 gene necessary for Caenorhabditis elegansvulval induction encodes a tyrosine kinase of the EGF receptor subfamily. Nature. 1990;348:693–699. - PubMed
- Balda MS, Anderson JM, Matter K. The SH3 domain of the tight junction protein ZO-1 binds to a serine protein kinase that phosphorylates a region COOH-terminal to this domain. FEBS Lett. 1996;399:326–332. - PubMed
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Ann Rev Cell Biol. 1992;8:365–393. - PubMed
- Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Ann Rev Physiol. 1995;57:417–445. - PubMed
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide Synthase with the postsynaptic density protein PSD95 and α1-syntrophin mediated by PDZ domains. Cell. 1996a;84:757–767. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases