Involvement of receptor-like protein tyrosine phosphatase zeta/RPTPbeta and its ligand pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) in neuronal migration - PubMed (original) (raw)
Involvement of receptor-like protein tyrosine phosphatase zeta/RPTPbeta and its ligand pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) in neuronal migration
N Maeda et al. J Cell Biol. 1998.
Abstract
Pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) is a specific ligand of protein tyrosine phosphatase zeta (PTPzeta)/receptor-like protein tyrosine phosphatase beta (RPTPbeta) expressed in the brain as a chondroitin sulfate proteoglycan. Pleiotrophin and PTPzeta isoforms are localized along the radial glial fibers, a scaffold for neuronal migration, suggesting that these molecules are involved in migratory processes of neurons during brain development. In this study, we examined the roles of pleiotrophin-PTPzeta interaction in the neuronal migration using cell migration assay systems with glass fibers and Boyden chambers. Pleiotrophin and poly-L-lysine coated on the substratums stimulated cell migration of cortical neurons, while laminin, fibronectin, and tenascin exerted almost no effect. Pleiotrophin-induced and poly-L-lysine-induced neuronal migrations showed significant differences in sensitivity to various molecules and reagents. Polyclonal antibodies against the extracellular domain of PTPzeta, PTPzeta-S, an extracellular secreted form of PTPzeta, and sodium vanadate, a protein tyrosine phosphatase inhibitor, added into the culture medium strongly suppressed specifically the pleiotrophin-induced neuronal migration. Furthermore, chondroitin sulfate C but not chondroitin sulfate A inhibited pleiotrophin-induced neuronal migration, in good accordance with our previous findings that chondroitin sulfate constitutes a part of the pleiotrophin-binding site of PTPzeta, and PTPzeta-pleiotrophin binding is inhibited by chondroitin sulfate C but not by chondroitin sulfate A. Immunocytochemical analysis indicated that the transmembrane forms of PTPzeta are expressed on the migrating neurons especially at the lamellipodia along the leading processes. These results suggest that PTPzeta is involved in the neuronal migration as a neuronal receptor of pleiotrophin distributed along radial glial fibers.
Figures
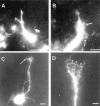
Figure 1
Pleiotrophin induces cell migration of cortical neurons. (A) Time-lapse video microscopy revealed several neurons (indicated by filled dots) migrating on a PTN fiber. Both neurons migrated ∼25 μm along the fiber during a 2-h observation. The cell located in the middle appeared to stay in the stationary period. (B) The percentage distributions of migration rates of cortical neurons on PLL (a), PTN (b) and LN (c) fibers are shown. On PLL fibers, 22% of the cells migrated at >4 μm/h, and the average speed of migration among them was 9.5 μm/h. On PTN fibers, 48% of the cells migrated at more than 4 μm/h, and their average speed of migration was 11.0 μm/h. On LN fibers, 18% (13%) of the cells migrated at >4 μm/h, and their average speed was 26.3 μm/h (6.8 μm/h); the values in parentheses are those when the rapidly migrating population (>36 μm/h) was not included. Bar, 10 μm.
Figure 2
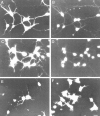
Neurons migrated on the pleiotrophin-coated Boyden chamber membranes. (A) A spindle-shaped neuron (large arrowhead) passing through a pore (arrow) of the filter had a leading process (small arrowheads). (B) After migration to the lower side, neurons resumed a rounded shape and extended long axon-like processes (arrowheads). In these samples, the migrated neurons were immunostained with anti-MAP2, after nonmigratory cells on the upper surface of the filters were removed by wiping with a cotton-tip applicator. Bar, 20 μm.
Figure 3
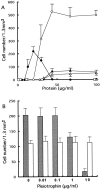
Boyden chamber cell migration of cortical neurons. (A) Cortical neurons were analyzed by Boyden chamber cell migration assay using membranes coated with various concentrations of pleiotrophin (○), poly
-l-
lysine (•), fibronectin (▴), laminin (▵), tenascin (□) and PTPζ-S (⋄). Pleiotrophin and poly
-l-
lysine significantly stimulated neuronal migration, whereas the other proteins exhibited low activities. Each point represents the mean ± SD of triplicate values. (B) Cortical neurons were subjected to Boyden chamber cell migration assay using membranes coated with 30 μg/ml pleiotrophin (shaded column) or 15 μg/ml poly
-l-
lysine (open column) in the presence of various concentrations of soluble pleiotrophin in the lower chamber. Soluble pleiotrophin suppressed the pleiotrophin-induced neuronal migration, but not the poly
-l-
lysine-induced migration. Each bar represents the mean ± SD of quintuplicate values.
Figure 4
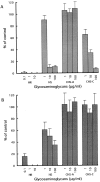
Effects of glycosaminoglycans on pleiotrophin-induced neuronal migration. Cortical neurons were analyzed by Boyden chamber cell migration assay using pleiotrophin-coated (A) and poly
-l-
lysine–coated (B) membranes. Neurons were cultured in the presence of heparin (HR), heparan sulfate (HS), chondroitin sulfate A (CHS-A) and chondroitin sulfate C (CHS-C). Heparin and heparan sulfate suppressed both pleiotrophin- and poly
-l-
lysine–induced neuronal migration. In contrast, chondroitin sulfate C inhibited only pleiotrophin-induced neuronal migration. Each bar represents the mean ± SD of triplicate values.
Figure 5
Effects of PTPζ-S on the pleiotrophin-induced neuronal migration. Boyden chamber cell migration assay was performed using (A) pleiotrophin- and (B) poly
-l-
lysine–coated membranes in the presence of various concentrations of PTPζ-S added into the culture medium. PTPζ-S significantly inhibited pleiotrophin-induced neuronal migration, whereas poly
-l-
lysine–induced migration was not affected. Each bar represents the mean ± SD of quintuplicate values.
Figure 5
Effects of PTPζ-S on the pleiotrophin-induced neuronal migration. Boyden chamber cell migration assay was performed using (A) pleiotrophin- and (B) poly
-l-
lysine–coated membranes in the presence of various concentrations of PTPζ-S added into the culture medium. PTPζ-S significantly inhibited pleiotrophin-induced neuronal migration, whereas poly
-l-
lysine–induced migration was not affected. Each bar represents the mean ± SD of quintuplicate values.
Figure 6
Polyclonal antibodies against PTPζ inhibit pleiotrophin-induced neuronal migration. Boyden chamber cell migration assay was performed using pleiotrophin- (shaded column) and poly
-l-
lysine– (open column) coated membranes in the presence of rabbit IgG (IgG), anti-6B4 PG (α6B4PG) and anti-31-5 (α31-5). Anti-6B4 PG inhibited pleiotrophin-induced neuronal migration, whereas poly
-l-
lysine–induced migration was not affected. Each bar represents the mean ± SD of triplicate values.
Figure 7
Effects of vanadate and protein tyrosine kinase inhibitors on the morphological differentiation of cortical neurons. Cortical neurons were cultured on pleiotrophin-coated coverslips in the presence of 0.02% DMSO (A and B), 100 μM sodium vanadate (C and D), 2 μg/ml herbimycin A (E), and 1 μg/ml erbstatin analogue (F). After 20 h in vitro, the neurons were double-immunostained with anti-MAP2 (A, C, E, and F) and anti-NFH (B and D). Arrowheads in B indicate the NFH-positive and MAP2-negative process. Bar, 20 μm.
Figure 8
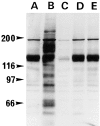
Analysis of protein tyrosine phosphorylation in cortical neurons treated with vanadate and kinase inhibitors. Cortical neurons were cultured for 20 h in the absence (A) or presence of 100 μM sodium vanadate (B), 1 μg/ml herbimycin A (C), 3 μg/ml lavendustin A (D), and 0.3 μg /ml erbstatin analogue (E). Cells were solubilized, and the proteins were separated by 7.5% SDS-PAGE and analyzed by immunoblotting with anti-phosphotyrosine monoclonal antibody 4G10. The positions of molecular mass markers (in kD) are shown on the left.
Figure 9
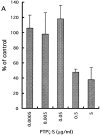
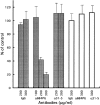
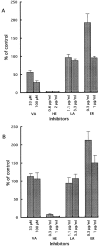
Effects of vanadate and protein tyrosine kinase inhibitors on neuronal migration. Boyden chamber cell migration assay was performed using the membranes coated with pleiotrophin (A) or poly
-l-
lysine (B) in the presence of sodium vanadate (VA), herbimycin A (HE), lavendustin A (LA), and erbstatin analogue (ER). Sodium vanadate specifically suppressed the pleiotrophin-induced neuronal migration. On the other hand, tyrosine kinase inhibitors exerted essentially the same effects on both pleiotrophin- and poly
-l-
lysine–induced neuronal migration. Each bar represents the mean ± SD of triplicate values.
Figure 10
Presence of transmembrane forms of PTPζ in cortical neurons. (A and B) Cortical neurons migrating on the pleiotrophin-coated filters (the underside) were fixed and immunostained with anti-RPTPβ, which recognizes intracellular D2 domain of PTPζ. An arrow in A points the position of a pore on the membrane, through which a neuron was migrating. The immunostaining was distributed broadly on neurons. Lamellipodia extended around the leading process were also stained with anti-RPTPβ (B, arrows). (C and D) Cortical neurons were cultured for 20 h on poly
-l-
lysine-coated coverslips, fixed and immunostained with anti-RPTPβ. Most cortical neurons were stained with anti-RPTPβ, although expression levels were variable. Cell bodies and neurites were intensely stained. At the growth cones, a subset of filopodias (C, arrow) and the rim of lamellipodia (D, arrowheads) were stained. When preimmune IgG was used instead of anti-RPTPβ, only weak background staining was observed (data not shown). Bars: (A, B, and D) 5 μm; (C) 10 μm.
Figure 11
Immunohistochemical localization of PTPζ in the developing cerebral cortex. (A and B) Frontal sections from E18 rat brains were immunohistochemically stained with anti-RPTPβ which recognizes transmembrane forms of PTPζ. Immunostainings were observed at all the layers including marginal zone (M), cortical plate (CP), subplate (SP), and intermediate zone (IZ). Higher magnification figure of cortical plate shows that the stainings were distributed along the cell surface of neurons (B, arrows) and a subset of neurons also displayed intracellular reticular stainings (B, arrowheads). (C) Strong mAb 6B4 immunostaining, which mainly corresponds to the presence of PTPζ-S, was observed on the marginal zone. In the superior part of cortical plate, the stainings were observed along radial glial fibers (C, arrowheads), and in the inferior part, surroundings of neurons were also stained (C, arrows). Bars: (A) 50 μm; (B) 10 μm; (C) 20 μm.
Similar articles
- A receptor-like protein-tyrosine phosphatase PTPzeta/RPTPbeta binds a heparin-binding growth factor midkine. Involvement of arginine 78 of midkine in the high affinity binding to PTPzeta.
Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. Maeda N, et al. J Biol Chem. 1999 Apr 30;274(18):12474-9. doi: 10.1074/jbc.274.18.12474. J Biol Chem. 1999. PMID: 10212223 - A chondroitin sulfate proteoglycan PTPzeta /RPTPbeta regulates the morphogenesis of Purkinje cell dendrites in the developing cerebellum.
Tanaka M, Maeda N, Noda M, Marunouchi T. Tanaka M, et al. J Neurosci. 2003 Apr 1;23(7):2804-14. doi: 10.1523/JNEUROSCI.23-07-02804.2003. J Neurosci. 2003. PMID: 12684467 Free PMC article. - [Mechanisms for dendritic morphogenesis of cerebellar purkinje cells: role of receptor-type protein tyrosine phosphatase zeta].
Tanaka M. Tanaka M. Nihon Shinkei Seishin Yakurigaku Zasshi. 2007 Jun;27(3):135-40. Nihon Shinkei Seishin Yakurigaku Zasshi. 2007. PMID: 17633525 Review. Japanese. - Heparin-binding proteins HB-GAM (pleiotrophin) and amphoterin in the regulation of cell motility.
Rauvala H, Huttunen HJ, Fages C, Kaksonen M, Kinnunen T, Imai S, Raulo E, Kilpeläinen I. Rauvala H, et al. Matrix Biol. 2000 Sep;19(5):377-87. doi: 10.1016/s0945-053x(00)00084-6. Matrix Biol. 2000. PMID: 10980414 Review.
Cited by
- Midkine and cytoplasmic maturation of mammalian oocytes in the context of ovarian follicle physiology.
Ikeda S, Yamada M. Ikeda S, et al. Br J Pharmacol. 2014 Feb;171(4):827-36. doi: 10.1111/bph.12311. Br J Pharmacol. 2014. PMID: 23889362 Free PMC article. Review. - Gene expression changes in the course of neural progenitor cell differentiation.
Gurok U, Steinhoff C, Lipkowitz B, Ropers HH, Scharff C, Nuber UA. Gurok U, et al. J Neurosci. 2004 Jun 30;24(26):5982-6002. doi: 10.1523/JNEUROSCI.0809-04.2004. J Neurosci. 2004. PMID: 15229246 Free PMC article. - Tumor-derived extracellular fragments of receptor protein tyrosine phosphatases (RPTPs) as cancer molecular diagnostic tools.
Craig SE, Brady-Kalnay SM. Craig SE, et al. Anticancer Agents Med Chem. 2011 Jan;11(1):133-40. doi: 10.2174/187152011794941244. Anticancer Agents Med Chem. 2011. PMID: 21235433 Free PMC article. Review. - Functions of chondroitin sulfate and heparan sulfate in the developing brain.
Maeda N, Ishii M, Nishimura K, Kamimura K. Maeda N, et al. Neurochem Res. 2011 Jul;36(7):1228-40. doi: 10.1007/s11064-010-0324-y. Epub 2010 Nov 26. Neurochem Res. 2011. PMID: 21110089 Review. - Sugar-dependent modulation of neuronal development, regeneration, and plasticity by chondroitin sulfate proteoglycans.
Miller GM, Hsieh-Wilson LC. Miller GM, et al. Exp Neurol. 2015 Dec;274(Pt B):115-25. doi: 10.1016/j.expneurol.2015.08.015. Epub 2015 Aug 24. Exp Neurol. 2015. PMID: 26315937 Free PMC article.
References
- Barnea G, Grumet M, Milev P, Silvennoinen O, Levy JB, Sap J, Schlessinger J. Receptor tyrosine phosphatase β is expressed in the form of proteoglycan and binds to the extracellular matrix protein tenascin. J Biol Chem. 1994;269:14349–14352. - PubMed
- Cannol PD, Barnea G, Levy JB, Sap J, Ehrlich M, Silvennoinen O, Schlessinger J, Musacchio JM. The expression of a novel receptor-type tyrosine phosphatase suggests a role in morphogenesis and plasticity of the nervous system. Dev Brain Res. 1993;75:293–298. - PubMed
- Cannol PD, Petanceska S, Schlessinger J, Musacchio JM. Three forms of RPTP-β are differentially expressed during gliogenesis in the developing rat brain and during glial cell differentiation in culture. J Neurosci Res. 1996;44:199–215. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases