Hemidesmosome formation is initiated by the beta4 integrin subunit, requires complex formation of beta4 and HD1/plectin, and involves a direct interaction between beta4 and the bullous pemphigoid antigen 180 - PubMed (original) (raw)
Hemidesmosome formation is initiated by the beta4 integrin subunit, requires complex formation of beta4 and HD1/plectin, and involves a direct interaction between beta4 and the bullous pemphigoid antigen 180
R Q Schaapveld et al. J Cell Biol. 1998.
Abstract
Hemidesmosomes (HDs) are stable anchoring structures that mediate the link between the intermediate filament cytoskeleton and the cell substratum. We investigated the contribution of various segments of the beta4 integrin cytoplasmic domain in the formation of HDs in transient transfection studies using immortalized keratinocytes derived from an epidermolysis bullosa patient deficient in beta4 expression. We found that the expression of wild-type beta4 restored the ability of the beta4-deficient cells to form HDs and that distinct domains in the NH2- and COOH-terminal regions of the beta4 cytoplasmic domain are required for the localization of HD1/plectin and the bullous pemphigoid antigens 180 (BP180) and 230 (BP230) in these HDs. The tyrosine activation motif located in the connecting segment (CS) of the beta4 cytoplasmic domain was dispensable for HD formation, although it may be involved in the efficient localization of BP180. Using the yeast two-hybrid system, we could demonstrate a direct interaction between beta4 and BP180 which involves sequences within the COOH-terminal part of the CS and the third fibronectin type III (FNIII) repeat. Immunoprecipitation studies using COS-7 cells transfected with cDNAs for alpha6 and beta4 and a mutant BP180 which lacks the collagenous extracellular domain confirmed the interaction of beta4 with BP180. Nevertheless, beta4 mutants which contained the BP180-binding region, but lacked sequences required for the localization of HD1/plectin, failed to localize BP180 in HDs. Additional yeast two- hybrid assays indicated that the 85 COOH-terminal residues of beta4 can interact with the first NH2-terminal pair of FNIII repeats and the CS, suggesting that the cytoplasmic domain of beta4 is folded back upon itself. Unfolding of the cytoplasmic domain may be part of a mechanism by which the interaction of beta4 with other hemidesmosomal components, e.g., BP180, is regulated.
Figures
Figure 10
Survey of the sites of interaction between the cytoplasmic domains of β4 and BP180. Yeast strain PJ69-4A was cotransformed with pAS2-BP180 and one of each of the listed pACT2-β4 constructs, or with an empty pACT2. Transformation mixtures were spread on SC-LT and SC-LTHA plates and grown for 9 d at 30°C. Plating efficiency on SC-LTHA plates is expressed relative to that on SC-LT plates of the same transformation. ++, \>50%; ±, 5–25%; and −, 0% indicate relative efficiencies, respectively. Plates were scored after 4 and 9 d of growth. Plating efficiencies above 25% represent fast-growing colonies that could be scored after 4 d; plating efficiencies lower than 25% represent colonies that clearly grow more slowly and could only be scored after 9 d of growth. All efficiencies listed represent an average of multiple independent transformation experiments on at least three separate days. Cotransformation efficiencies (on SC-LT plates) for all plasmid combinations listed were always at least 104 cfu/μg, and the difference between the various β4 plasmids tested never was greater than twofold. Cotransformation of yeast PJ69-4A with empty pAS2-1 and pACT2 vectors never resulted in the growth of colonies on SC-LTHA plates, nor did cotransformation of the yeast strain with either the pAS2-BP180 plasmid and an empty pACT2 vector or any of the pACT2-β4 plasmids and an empty pAS2-1 vector, showing that none of the GAL4 fusion proteins encoded by these recombinant plasmids by themselves could cause activation of the His and Ade reporter genes.
Figure 12
Intramolecular interactions in the β4 cytoplasmic domain. Yeast strain PJ69-4A was cotransformed with two different β4 constructs as listed. Other details are as in Fig. 10, except that the efficiencies represent an average of multiple independent transformation experiments on at least two separate days.
Figure 1
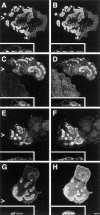
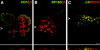
Immunolocalization of hemidesmosomal components in the NHK (A–E) and PA-JEB cell lines (F–J) by confocal laser microscopy. Cells were grown on glass coverslips in HAMF12/DME (1:3) medium, fixed, and immunolabeled using the rat mAb GoH3 directed against α6 (A and F), the mAb 450-9D against β4 (B and G), a rabbit anti-BP230 antiserum (C and H), the mAb 233 against BP180 (D and I), and the mAb 121 against HD1 (E and J). In NHK, the hemidesmosomal components are concentrated at sites of cell– substrate contact in patches characteristic for HD-like structures. In PA-JEB keratinocytes, only BP230 (H) and BP180 (I) are found concentrated in the rare HD-like structures in fewer than 1% of the cells. α6 is colocalized with vinculin (data not shown) in dots and streaks representing focal adhesions (F), whereas HD1/plectin is found diffusely distributed throughout the cell (J). No β4 reactivity is observed in PA-JEB cells (G). Sections were focused at the cell–substrate interface. Arrowheads, positions from which the perpendicular sections, shown in the insets, were taken. Bar, 10 μm.
Figure 2
Immunoprecipitation of integrin complexes and BP180 from NHK and PA-JEB keratinocytes. Lysates of 125I-labeled NHK (left) and PA-JEB keratinocytes (right) were immunoprecipitated with the mAbs P1E6 (against α2, lane 1), J143 (α3, lane 2), Sam-1 (α5, lane 3), NKI-M9 (αv, lane 4), J8H (α6, lane 5), TS2/16 (β1, lane 6), 450-9D (β4, lane 7), 439-9B (β4, lane 8, right) and 1D1 (BP180, lane 8, left and lane 9, right, respectively). The antibody against α6 precipitated this subunit associated with β4 from NHK, whereas from the PA-JEB cells α6 and β1 were precipitated, but not β4. The faint band which migrates just above β1 and seen in the lanes containing the anti-β4 immunoprecipitates from PA-JEB cells, represents a nonspecific product. β1 is found in association with α2, α3, α5, and α6 in PA-JEB cells, but only with α2, α3, and α5 in NHK. Precipitation of β1 with α5 is evident after prolonged exposure (data not shown). Samples were analyzed on a SDS-polyacrylamide (5%) gel under nonreducing conditions. The positions of molecular weight standards (in kD) are indicated on the left.
Figure 3
Expression of full-length, TAM-mutated, COOH-terminally truncated (A), and internal deletion mutant (B) β4 cDNAs in PA-JEB keratinocytes. Schematic representation of the cDNA constructs encoding wild-type and mutant forms of β4 carrying deletions of the cytoplasmic domain. Boxes, FNIII repeats in which the number of the repeat is shown; triangle, the β4B-specific insert of 53 amino acids in the CS (A), or the 17-amino acid deletion in the second FNIII repeat as described for a PA-JEB patient (Vidal et al., 1995) (B). Y > F mutations of the TAM are represented by F. The immunolocalizations of the hemidesmosomal components HD1/plectin, BP180, and BP230 were investigated upon transfection of the β4 constructs in PA-JEB cells. Colocalization of HD1/plectin, BP180, and BP230, respectively, with α6β4 at the basal cell surface in HD-like structures is observed in ++, 75–100%; +, 25–75%; ±, 1–25%; −, 0%, of the β4-transfected cells.
Figure 4
Immunoprecipitation analysis of PA-JEB keratinocytes transfected with cDNA encoding wild-type β4 (PA-JEB R). Lysates of [35S]methionine/cysteine-labeled NHK and PA-JEB cells were immunoprecipitated with the mAb J8H against α6 (lane 1), the anti-β4 mAb 450-9D (lane 2), and the mAb TS2/16 against β1 (lane 3). Antibodies against α6 precipitate α6 and β4 from NHK, and α6 together with β1 from PA-JEB cells. Antibodies against β4 precipitate this subunit together with α6 from NHK, but not from PA-JEB cells. In contrast, from PA-JEB cells transfected with cDNA for β4 (PA-JEB R) antibodies against β4 (lane 1) precipitate β4 together with α6. Coprecipitation of α6 is evident after prolonged exposure (lane 2). Samples were analyzed on a SDS-polyacrylamide (5%) gel under nonreducing conditions. The positions of molecular weight standards (in kD) are indicated on the left and those of the β4 and α6 integrin subunits are indicated on the right.
Figure 5
Expression of wild-type β4 in PA-JEB keratinocytes induces the formation of HD-like structures. PA-JEB cells were transfected with cDNA encoding β4A. After 36 h, cells were fixed, permeabilized, and subjected to double labeling immunofluorescence for β4 (A–E) and α6 (F), vinculin (G), HD1/plectin (H), BP180 (I), and BP230 (J). Upon transfection, expression of β4 results in the formation of HD-like structures, in which β4 is concentrated at sites of cell–substrate contact and codistributed with α6, HD1/plectin, BP180, and BP230 (insets are the perpendicular sections). In cells expressing β4, α6 is now found in HD-like structures (F), and no longer concentrated in focal adhesions at the outer periphery of these structures, as shown by staining for vinculin (G). Bar, 10 μm.
Figure 6
Expression of a β4 TAM mutant induces the assembly of HD-like structures in transfected PA-JEB keratinocytes. PA-JEB cells transfected with TAM-mutated β4A cDNA were double stained for β4 (A, C, E, and G) and α6 (B), HD1/plectin (D), BP180 (F), and BP230 (H). As shown in the perpendicular sections, a β4 molecule with phenylalanine substitutions at the TAM becomes localized together with α6 at the basal cell side and recruits HD1/plectin, BP180, and BP230 to sites of cell–substrate contact. The redistribution of BP180 was, however, slightly impaired. Bar, 10 μm.
Figure 7
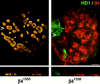
A segment comprising the first pair of FNIII repeats and a 27-amino acid stretch of the CS is essential for the localization of HD1/plectin at the basal cell surface. Representatives of double immunofluoresence analyses of PA-JEB cells transfected with cDNA encoding COOH-terminal deletion mutants of β4 as depicted in Fig. 3 are shown. PA-JEB cells transfected with cDNA coding for β41,355 or β41,328 were immunolabeled with antibodies against β4 (red) and HD1 (green). Although β41,355 still induces the redistribution of HD1/plectin to the basal surface of the cell (_lef_t), β41,328, lacking an additional 27 amino acids of the CS does not affect the distribution of HD1/plectin (right). Noteworthy, the distribution pattern of α6β4 in the absence of HD1/plectin is comparable to that of α6β4 together with HD1/plectin and indistinguishable by confocal microscopy. Bar, 10 μm.
Figure 8
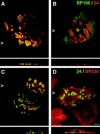
Distribution of BP180 and BP230 in PA-JEB keratinocytes is affected by COOH-terminal truncations of β4. PA-JEB cells transfected with cDNA encoding mutant forms of β4 were double-stained for β4 (red) and BP180 (green) (A and B) or for β4 (green) and BP230 (red) (C and D). Shown are representatives of transfections with β41,436 cDNA. Truncation of the second pair of FNIII repeats already impairs the recruitment of BP180 (B) and BP230 (D) to the basal cell surface, although cells showing colocalization of the BP antigens with β4 can readily be found (A and C). Increasing COOH-terminal truncations further impair the localization of BP180 and BP230 at the basal cell side (refer to Fig. 3_A_). In cells transfected with β41,328 cDNA, which also do not show basal localization of HD1/plectin, BP180 and BP230 remain diffusely distributed throughout the cell (data not shown). Bar, 10 μm.
Figure 9
Coimmunoprecipi-tation of _β_4 and BP180. Lysates of COS-7 cells cotransfected with cDNAs for _α_6A and wild-type _β_4A, _β_41,355, or _β_41,328 as well as an empty pCI-Neo vector or a pCI-Neo construct encoding the BP180 cytoplasmic domain were subjected to immunoprecipitation with a mixture (1:1:1) of three anti-_β_4 mAbs, 4.3E1, 113C and 450-9D, respectively, or with the mAb FLAG™ M2. Samples were resolved on a SDS-polyacrylamide (8%) gel under nonreducing conditions. Shown is an immunoblot analysis developed with the rabbit polyclonal anti-serum against _β_4 (top) and the mAb FLAG™ M2 to detect BP180 (bottom) among the immunoprecipitated proteins. When samples immunoprecipitated with the anti-_β_4 mAbs were subjected to immunoblotting with the mAb FLAG™ M2 (bottom) or a polyclonal anti-BP180 antiserum (data not shown), the mutant form of BP180 was not detectable in the anti-_β_4 immunoprecipitates. The positions of molecular weight standards (in kD) are indicated on the right.
Figure 11
Basal localization of HD1/plectin together with β4 is essential for the recruitment of BP180. PA-JEB cells transfected with cDNA encoding internal deletion mutants of β4 were double-stained for (A) β4 (red) and HD1/plectin (green), (B) β4 (red) and BP180 (green), and (C) β4 (green) and BP230 (red). Shown are representatives of transfections with β4AΔ1,249–1,265. Deletion of 17 amino acids in the second FNIII repeat or its complete deletion prevent the recruitment of HD1/plectin (A). As a consequence, α6β4 clustered at the basal cell surface is no longer capable of recruiting BP180 to these HD1/plectin-lacking clusters although the binding sites for BP180 are still present (B). In a few cases (i.e., <25% of β4-transfected cells) these β4 mutants were able to recruit BP230 in an HD1/plectin- and BP180-independent manner to the basal cell surface (C). Bar, 10 μm.
Similar articles
- Analysis of the interactions between BP180, BP230, plectin and the integrin alpha6beta4 important for hemidesmosome assembly.
Koster J, Geerts D, Favre B, Borradori L, Sonnenberg A. Koster J, et al. J Cell Sci. 2003 Jan 15;116(Pt 2):387-99. doi: 10.1242/jcs.00241. J Cell Sci. 2003. PMID: 12482924 - The localization of bullous pemphigoid antigen 180 (BP180) in hemidesmosomes is mediated by its cytoplasmic domain and seems to be regulated by the beta4 integrin subunit.
Borradori L, Koch PJ, Niessen CM, Erkeland S, van Leusden MR, Sonnenberg A. Borradori L, et al. J Cell Biol. 1997 Mar 24;136(6):1333-47. doi: 10.1083/jcb.136.6.1333. J Cell Biol. 1997. PMID: 9087447 Free PMC article. - Role of the bullous pemphigoid antigen 180 (BP180) in the assembly of hemidesmosomes and cell adhesion--reexpression of BP180 in generalized atrophic benign epidermolysis bullosa keratinocytes.
Borradori L, Chavanas S, Schaapveld RQ, Gagnoux-Palacios L, Calafat J, Meneguzzi G, Sonnenberg A. Borradori L, et al. Exp Cell Res. 1998 Mar 15;239(2):463-76. doi: 10.1006/excr.1997.3923. Exp Cell Res. 1998. PMID: 9521865 - Molecular complexity of the cutaneous basement membrane zone.
Uitto J, Pulkkinen L. Uitto J, et al. Mol Biol Rep. 1996;23(1):35-46. doi: 10.1007/BF00357071. Mol Biol Rep. 1996. PMID: 8983017 Review. - Advances and perspectives of the architecture of hemidesmosomes: lessons from structural biology.
de Pereda JM, Ortega E, Alonso-García N, Gómez-Hernández M, Sonnenberg A. de Pereda JM, et al. Cell Adh Migr. 2009 Oct-Dec;3(4):361-4. doi: 10.4161/cam.3.4.9525. Epub 2009 Oct 16. Cell Adh Migr. 2009. PMID: 19736524 Free PMC article. Review.
Cited by
- Compound Heterozygous Mutations with a Novel Variant in Integrin Beta4 Cause Epidermolysis Bullosa with Pyloric Atresia and Urologic Abnormalities.
Yoo DS, Lee SJ, Kim SE, Kim SC, Lee SE. Yoo DS, et al. Yonsei Med J. 2020 Sep;61(9):831-833. doi: 10.3349/ymj.2020.61.9.831. Yonsei Med J. 2020. PMID: 32882768 Free PMC article. No abstract available. - Binding of integrin alpha6beta4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding.
Geerts D, Fontao L, Nievers MG, Schaapveld RQ, Purkis PE, Wheeler GN, Lane EB, Leigh IM, Sonnenberg A. Geerts D, et al. J Cell Biol. 1999 Oct 18;147(2):417-34. doi: 10.1083/jcb.147.2.417. J Cell Biol. 1999. PMID: 10525545 Free PMC article. - The spectraplakin Dystonin antagonizes YAP activity and suppresses tumourigenesis.
Jain PB, Guerreiro PS, Canato S, Janody F. Jain PB, et al. Sci Rep. 2019 Dec 27;9(1):19843. doi: 10.1038/s41598-019-56296-z. Sci Rep. 2019. PMID: 31882643 Free PMC article. - Deciphering the Contribution of BP230 Autoantibodies in Bullous Pemphigoid.
Cole C, Borradori L, Amber KT. Cole C, et al. Antibodies (Basel). 2022 Jun 28;11(3):44. doi: 10.3390/antib11030044. Antibodies (Basel). 2022. PMID: 35892704 Free PMC article. Review.
References
- Baker SE, Skalli O, Goldman RD, Jones JCR. Laminin-5 and modulation of keratin cytoskeleton arrangement in FG pancreatic carcinoma cells: involvement of IFAP300/HD1 and evidence that laminin-5/cell interactions correlate with a dephosphorylation of α6A integrin. Cell Motil Cytoskeleton. 1997;36:271–286. - PubMed
- Borradori L, Sonnenberg A. Hemidesmosomes: roles in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647–656. - PubMed
- Borradori L, Chavanas S, Schaapveld RQJ, Gagnoux-Palacios L, Calafat J, Meneguzzi G, Sonnenberg A. Role of the bullous pemphigoid antigen 180 (BP180) in the assembly of hemidesmosomes and cell adhesion. Reexpression of BP180 in generalized atrophic benign epidermolysis bullosa keratinocytes. Exp Cell Res. 1998;239:463–476. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous