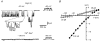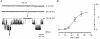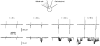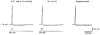Na+-activated K+ channels in small dorsal root ganglion neurones of rat - PubMed (original) (raw)
Na+-activated K+ channels in small dorsal root ganglion neurones of rat
U Bischoff et al. J Physiol. 1998.
Abstract
1. Whole-cell Na+-activated K+ (KNa) channel currents and single KNa channels were studied with the patch-clamp method in small (20-25 micrometer) dorsal root ganglion (DRG) neurones in slices of rat dorsal root ganglia. 2. The whole-cell KNa channel current was identified as an additional K+-selective leakage current which appeared after cell perfusion with internal solutions containing different [Na+]. The concentration for half-maximal activation of KNa channel current was 39 mM and the Hill coefficient was 3.5. At [Na+]i above 12 mM, KNa channel current dominated the unspecific leakage current. The ratio of maximum KNa channel current to unspecific leakage current was 45. 3. KNa channel current was not activated by internal Li+. It was suppressed by external 20 mM Cs+ but not by 10 mM tetraethylammonium. 4. Single KNa channels with a conductance of 142 pS in 155 mM external K+ (K+o)-85 mM internal K+ (K+i) solutions were observed at a high density of about 2 channels micrometer-2. 5. In two-electrode experiments, a direct correlation was seen between development of whole- cell KNa channel current and activation of single KNa channels during perfusion of the neurone with Na+-containing internal solution. 6. Under current-clamp conditions, KNa channels did not contribute to the action potential. However, internal perfusion of the neurone with Na+ shifted the resting potential towards the equilibrium potential for K+ (EK). Varying external [K+] indicated that in neurones perfused with Na+-containing internal solution the resting potential followed the EK values predicted by the Nernst equation over a broader voltage range than in neurones perfused with Na+-free solution. 7. It is concluded that the function of KNa channels has no links to firing behaviour but that the channels could be involved in setting or stabilizing the resting potential in small DRG neurones.
Figures
Figure 1. Na+-activated leakage currents in small DRG neurones
A, whole-cell leakage currents activated by 150 ms hyperpolarizing voltage steps to -120 mV from a holding potential of -80 mV. Recordings were made from three different neurones using pipettes filled with 0, 20 and 70 m
m
Nai+ solutions (indicated near the traces). B, dependence of the amplitude of the leakage currents on [Na+]i. The leakage currents were measured at the end of the hyperpolarizing pulse. Each point represents the mean leakage current (±
s.e.m.
, where the error bar exceeds the symbol size) measured in five different neurones. It was supposed that the total leakage current, _I_L, consisted of a Na+-independent (unspecific) component, _I_L(0), and a Na+-activated component, _I_L(Na): _I_L = _I_L(0)+_I_L(Na). The fitting procedure is described in the text. C, _I_L(0) component (dashed line) and fitted curve for the _I_L(Na) component as a function of [Na+]i. D, leakage currents activated by a 150 ms voltage pulse from -80 to -120 mV in Ca2+-free solution (Control) and after addition of external 20 m
m
Cs+ and 10 m
m
TEA. Recordings were obtained from the same neurone perfused with 70 m
m
Nai+ solution. E, leakage currents recorded from two neurones using pipettes filled with 70 m
m
Nai+ and 70 m
m
Lio+ solutions.
Figure 2
Experiments with double-solution pipette_A_, leakage currents activated by voltage pulses from -80 to -120 mV at different times after membrane breakthrough and establishment of the whole-cell recording mode. The tip of the double-solution pipette was filled with 70 m
m
Nai+ solution and the rest of the pipette with 0 m
m
Nai+ solution. A schematic drawing is shown at the top. B, amplitude of the leakage current as a function of time elapsed after membrane breakthrough. Data points during the first 2 min are given at a higher time resolution. Filled symbols correspond to original recordings shown in A. Kinetics of ‘recovery’ were fitted by a mono-exponential function with a time constant of 15 min. C, leakage currents recorded with a standard pipette filled with 0 m
m
Nai+ solution only. D, development of the leakage current during an experiment with a standard pipette containing 70 m
m
Nai+ only.
Figure 3. Single KNa channel currents
A, single-channel currents recorded from inside-out patches using pipettes filled with high-Ko+ and Ca2+-freeo solutions in 70 m
m
Nai+ bath solution. Holding potentials are indicated near the corresponding traces. Part of the recording is shown at a higher time resolution. Several substates are indicated by asterisks. B, _i_- V plots in high-Ko+ (•; 4 patches) and Ca2+-freeo solutions (^; 4 patches). The arrows indicate the theoretical _E_K for both solutions.
Figure 4. Effect of internal [Na+] on KNa channel activation
A, recordings of single KNa channels in 0, 20 and 70 m
m
Nai+ solutions. Holding potential was -60 mV. Inside-out patch; high-Ko+ pipette solution. B, open probability (_P_o) as a function of [Nai+. _P_o was calculated from 1-2 min recordings at a membrane potential of -60 mV. Each point represents the mean (±
s.e.m.
, where the error bar exceeds the symbol size) of measurements from five inside-out patches. The data points were fitted with the equation: _P_o = _P_o(max)/(1 + (EC50/[Na+]i)_n_H), where _P_o(max) (maximum _P_o) is 0.37 ± 0.03, EC50 is 47.9 ± 2.3 m
m
and _n_H (Hill coefficient) is 4.4.
Figure 5. KNa channel activity in intact neurones
Cell-attached recordings of channel activity in three different neurones. Recordings from three membrane patches before (cell attached; left) and after their excision (inside out; right). Pipette potential was 0 mV during cell-attached recording and -80 mV in inside-out mode. The pipettes contained high-Ko+-TEA solution. For recording in the inside-out mode the patches were transferred to a bath containing 70 m
m
Nai+ solution.
Figure 6. Simultaneous recordings of leakage and single KNa channel currents
Simultaneous recordings of Na+-activated leakage current (top traces) and single KNa channel current (bottom traces) with two electrodes. The electrode used in whole-cell mode contained 70 m
m
Nai+ solution. Holding potential was -80 mV and the leakage currents were activated each second by 60 ms pulses to -120 mV. The time elapsed (t) after membrane breakthrough is indicated above the traces. The resistance of the whole-cell pipette was 6 MΩ. The pipette used for cell-attached recordings was filled with high-Ko+-TEA solution and had a resistance of 5 MΩ. The potential in the pipette during cell-attached recording was 0 mV with respect to the bath solution.
Figure 7. Influence of external Li+ on the shape of the action potential
Action potentials evoked by short (12.5 ms) current pulses of 240 pA in external Ca2+-free and 141 m
m
Lio+ solutions. Both action potentials are shown superimposed on the right. The membrane potential was kept at -70 mV in both solutions by injection of inward or outward steady-state currents. The pipette contained high-Ki+ solution.
Figure 8. Effect of internal Na+ on resting potential in small DRG neurones
A, resting potential in two different neurones shown as a function of time elapsed after membrane breakthrough. One neurone had a small original leakage current and a resting potential of -82 mV (^). Another neurone with a large original unspecific leakage current had a depolarized resting potential of -50 mV (•). The bath contained Ca2+-free solution and the pipette solution was 70 m
m
Nai+. The theoretical _E_K for external Ca2+-free-internal 70 m
m
Nai+ solutions (5.6 m
m
Ko+-85 m
m
Ki+) of -68 mV is indicated by the dashed line. The leakage currents recorded immediately after membrane breakthrough and several minutes after their saturation are shown in the insets. B, resting potential as a function of external K+ concentration measured in neurones perfused with 0 (•), 10 (▿) and 30 m
m
Nai+ (^) solutions. The straight line corresponds to the Nernst equation at 25 °C: 25.7 ln([K+]o/[K+]i), where the value given (25.7) is in mV and [K+]i is 85 m
m
.
Similar articles
- Single voltage-gated K+ channels and their functions in small dorsal root ganglion neurones of rat.
Safronov BV, Bischoff U, Vogel W. Safronov BV, et al. J Physiol. 1996 Jun 1;493 ( Pt 2)(Pt 2):393-408. doi: 10.1113/jphysiol.1996.sp021391. J Physiol. 1996. PMID: 8782104 Free PMC article. - Slack and Slick KNa channels are required for the depolarizing afterpotential of acutely isolated, medium diameter rat dorsal root ganglion neurons.
Gao SB, Wu Y, Lü CX, Guo ZH, Li CH, Ding JP. Gao SB, et al. Acta Pharmacol Sin. 2008 Aug;29(8):899-905. doi: 10.1111/j.1745-7254.2008.00842.x. Acta Pharmacol Sin. 2008. PMID: 18664322 - Properties and functions of Na(+)-activated K+ channels in the soma of rat motoneurones.
Safronov BV, Vogel W. Safronov BV, et al. J Physiol. 1996 Dec 15;497 ( Pt 3)(Pt 3):727-34. doi: 10.1113/jphysiol.1996.sp021803. J Physiol. 1996. PMID: 9003557 Free PMC article. - Spatial distribution of NA+ and K+ channels in spinal dorsal horn neurones: role of the soma, axon and dendrites in spike generation.
Safronov BV. Safronov BV. Prog Neurobiol. 1999 Oct;59(3):217-41. doi: 10.1016/s0301-0082(98)00051-3. Prog Neurobiol. 1999. PMID: 10465379 Review.
Cited by
- Emerging role of the KCNT1 Slack channel in intellectual disability.
Kim GE, Kaczmarek LK. Kim GE, et al. Front Cell Neurosci. 2014 Jul 28;8:209. doi: 10.3389/fncel.2014.00209. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25120433 Free PMC article. Review. - A biophysically comprehensive model of urothelial afferent neurons: implications for sensory signalling in urinary bladder.
Aruljothi S, Manchanda R. Aruljothi S, et al. J Comput Neurosci. 2024 Feb;52(1):21-37. doi: 10.1007/s10827-024-00865-3. Epub 2024 Feb 12. J Comput Neurosci. 2024. PMID: 38345739 - Cellular mechanisms of long-lasting adaptation in visual cortical neurons in vitro.
Sanchez-Vives MV, Nowak LG, McCormick DA. Sanchez-Vives MV, et al. J Neurosci. 2000 Jun 1;20(11):4286-99. doi: 10.1523/JNEUROSCI.20-11-04286.2000. J Neurosci. 2000. PMID: 10818164 Free PMC article. - Activation of DOR attenuates anoxic K+ derangement via inhibition of Na+ entry in mouse cortex.
Chao D, Bazzy-Asaad A, Balboni G, Salvadori S, Xia Y. Chao D, et al. Cereb Cortex. 2008 Sep;18(9):2217-27. doi: 10.1093/cercor/bhm247. Epub 2008 Jan 17. Cereb Cortex. 2008. PMID: 18203692 Free PMC article. - The N-terminal domain of Slack determines the formation and trafficking of Slick/Slack heteromeric sodium-activated potassium channels.
Chen H, Kronengold J, Yan Y, Gazula VR, Brown MR, Ma L, Ferreira G, Yang Y, Bhattacharjee A, Sigworth FJ, Salkoff L, Kaczmarek LK. Chen H, et al. J Neurosci. 2009 Apr 29;29(17):5654-65. doi: 10.1523/JNEUROSCI.5978-08.2009. J Neurosci. 2009. PMID: 19403831 Free PMC article.
References
- Bader CR, Bernheim L, Bertrand D. Sodium-activated potassium current in cultured avian neurones. Nature. 1985;317:540–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials