Macrophages in human atheroma contain PPARgamma: differentiation-dependent peroxisomal proliferator-activated receptor gamma(PPARgamma) expression and reduction of MMP-9 activity through PPARgamma activation in mononuclear phagocytes in vitro - PubMed (original) (raw)
Macrophages in human atheroma contain PPARgamma: differentiation-dependent peroxisomal proliferator-activated receptor gamma(PPARgamma) expression and reduction of MMP-9 activity through PPARgamma activation in mononuclear phagocytes in vitro
N Marx et al. Am J Pathol. 1998 Jul.
Abstract
Mononuclear phagocytes play an important role in atherosclerosis and its sequela plaque rupture in part by their secretion of matrix metalloproteinases (MMPs), including MMP-9. Peroxisomal proliferator-activated receptor gamma (PPARgamma), a transcription factor in the nuclear receptor superfamily, regulates gene expression in response to various activators, including 15-deoxy-delta12,14-prostaglandin J2 and the antidiabetic agent troglitazone. The role of PPARgamma in human atherosclerosis is unexplored. We report here that monocytes/macrophages in human atherosclerotic lesions (n = 12) express immunostainable PPARgamma. Normal artery specimens (n = 6) reveal minimal immunoreactive PPARgamma. Human monocytes and monocyte-derived macrophages cultured for 6 days in 5% human serum expressed PPARgamma mRNA and protein by reverse transcription-polymerase chain reaction and Western blotting, respectively. In addition, PPARgamma mRNA expression in U937 cells increased during phorbol 12-myristate 13 acetate-induced differentiation. Stimulation of PPARgamma with troglitazone or 15-deoxy-delta12,14-prostaglandin J2 in human monocyte-derived macrophages inhibited MMP-9 gelatinolytic activity in a concentration-dependent fashion as revealed by zymography. This inhibition correlates with decreased MMP-9 secretion as determined by Western blotting. Thus, PPARgamma is present in macrophages in human atherosclerotic lesions and may regulate expression and activity of MMP-9, an enzyme implicated in plaque rupture. PPARgamma is likely to be an important regulator of monocyte/macrophage function with relevance for human atherosclerotic disease.
Figures
Figure 1.
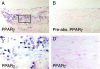
Expression of PPARγ in human atherosclerotic lesions. A: Low-power view of frozen section of human carotid lesions shows immunoreactive PPARγ next to the lipid core in the shoulder region with abundant macrophages present. Similar results were seen in other atherosclerotic specimens (n = 12). B: No immunoreactive PPARγ is detectable in parallel sections stained with PPARγ antibodies preabsorbed with the immunizing peptide, indicating that staining for PPARγ in (A) and (C) is specific. Similar results were seen with immunoglobulin G controls (not shown). C: High-power view of the area indicated by the rectangle in (A) shows PPARγ staining restricted to macrophage nuclei (see quantification in Results). D: In nonatherosclerotic arteries (n = 6), little PPARγ could be detected, with scant staining in occasional vascular smooth muscle cells.
Figure 2.
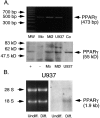
A: PPARγ mRNA and protein are expressed in cells of the monocyte/macrophage lineage. Upper panel: reverse transcription-polymerase chain reaction of PPARγ mRNA in freshly prepared monocytes (Mo), monocyte-derived macrophages (MØ), and PMA-differentiated U937 cells (U937) reveals a cDNA fragment of the expected size. Also shown are a 100-bp DNA ladder (MW) and negative control without cDNA (Co). Lower panel: Western blot analysis of PPARγ protein expression in nuclear extracts of freshly prepared monocytes (Mo), monocyte-derived macrophages (MØ), and U937 cells (U937) reveals a band of the appropriate size. The identity of this band is confirmed by co-migration with a band seen in PPARγ-transfected human skin fibroblasts (+) but not in similar but untransfected fibroblasts (−). All results shown were reproduced in three independent experiments. B (right): PPARγ mRNA expression in undifferentiated (Undiff.) and PMA-differentiated U937 cells (Diff.), as shown by Northern blot analysis. Differentiated U937 cells show increased PPARγ mRNA expression compared with undifferentiated cells. B (left): Ethidium bromide staining demonstrates equal loading of intact RNA. Results shown were reproduced in three independent experiments.
Figure 3.
A: PPARγ activators decrease MMP-9 gelatinolytic activity in supernatants from monocyte-derived macrophages (MØ) in a concentration-dependent fashion, as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis zymography. Monocytes were cultured in 5% human serum in the presence or absence of troglitazone or 15 d-PGJ2 as indicated. Supernatants of PMA-treated fibroblasts served as a control (Co). Gelatinolytic activity of freshly prepared monocytes is also shown (Mo). Equal amounts of lysates were loaded. Results shown were reproduced in three independent experiments. B: PPARγ activators decrease MMP-9 protein levels in supernatants from monocyte-derived macrophages (MØ). Vascular smooth muscle cells treated with PMA at 50 μg/L served as a control (Co). Supernatants from freshly prepared monocyte supernatant (Mo) are also shown. Similar results were reproduced in three independent experiments.
Similar articles
- Peroxisome proliferator-activated receptor gamma activators inhibit gene expression and migration in human vascular smooth muscle cells.
Marx N, Schönbeck U, Lazar MA, Libby P, Plutzky J. Marx N, et al. Circ Res. 1998 Nov 30;83(11):1097-103. doi: 10.1161/01.res.83.11.1097. Circ Res. 1998. PMID: 9831704 Free PMC article. - Troglitazone, a PPARgamma ligand, inhibits osteopontin gene expression in human monocytes/macrophage THP-1 cells.
Oyama Y, Kurabayashi M, Akuzawa N, Nagai R. Oyama Y, et al. J Atheroscler Thromb. 2000;7(2):77-82. doi: 10.5551/jat1994.7.77. J Atheroscler Thromb. 2000. PMID: 11426586 - Therapeutic potential of thiazolidinediones in activation of peroxisome proliferator-activated receptor gamma for monocyte recruitment and endothelial regeneration.
Tanaka T, Fukunaga Y, Itoh H, Doi K, Yamashita J, Chun TH, Inoue M, Masatsugu K, Saito T, Sawada N, Sakaguchi S, Arai H, Nakao K. Tanaka T, et al. Eur J Pharmacol. 2005 Jan 31;508(1-3):255-65. doi: 10.1016/j.ejphar.2004.10.056. Epub 2004 Dec 30. Eur J Pharmacol. 2005. PMID: 15680279 - Peroxisome proliferator-activated receptor gamma1 (PPARgamma1) expresses in rat mesangial cells and PPARgamma agonists modulate its differentiation.
Asano T, Wakisaka M, Yoshinari M, Iino K, Sonoki K, Iwase M, Fujishima M. Asano T, et al. Biochim Biophys Acta. 2000 Jun 2;1497(1):148-54. doi: 10.1016/s0167-4889(00)00054-9. Biochim Biophys Acta. 2000. PMID: 10838168 - PPARgamma activation induces the expression of the adipocyte fatty acid binding protein gene in human monocytes.
Pelton PD, Zhou L, Demarest KT, Burris TP. Pelton PD, et al. Biochem Biophys Res Commun. 1999 Aug 2;261(2):456-8. doi: 10.1006/bbrc.1999.1071. Biochem Biophys Res Commun. 1999. PMID: 10425206
Cited by
- Modulating peroxisome proliferator-activated receptors for therapeutic benefit? Biology, clinical experience, and future prospects.
Rosenson RS, Wright RS, Farkouh M, Plutzky J. Rosenson RS, et al. Am Heart J. 2012 Nov;164(5):672-80. doi: 10.1016/j.ahj.2012.06.023. Epub 2012 Oct 16. Am Heart J. 2012. PMID: 23137497 Free PMC article. Review. - Mitochondrial mitophagic mechanisms of myocardial matrix metabolism and remodelling.
Vacek TP, Vacek JC, Tyagi SC. Vacek TP, et al. Arch Physiol Biochem. 2012 Feb;118(1):31-42. doi: 10.3109/13813455.2011.635660. Epub 2011 Dec 19. Arch Physiol Biochem. 2012. PMID: 22181043 Free PMC article. Review. - PPAR Medicines and Human Disease: The ABCs of It All.
Apostoli AJ, Nicol CJ. Apostoli AJ, et al. PPAR Res. 2012;2012:504918. doi: 10.1155/2012/504918. Epub 2012 Aug 7. PPAR Res. 2012. PMID: 22919365 Free PMC article. - Peroxisome proliferator-activated receptor agonists: do they increase cardiovascular risk?
Aljada A, Shah KA, Mousa SA. Aljada A, et al. PPAR Res. 2009;2009:460764. doi: 10.1155/2009/460764. Epub 2009 Aug 19. PPAR Res. 2009. PMID: 19696948 Free PMC article. - Differential inhibition of macrophage foam-cell formation and atherosclerosis in mice by PPARalpha, beta/delta, and gamma.
Li AC, Binder CJ, Gutierrez A, Brown KK, Plotkin CR, Pattison JW, Valledor AF, Davis RA, Willson TM, Witztum JL, Palinski W, Glass CK. Li AC, et al. J Clin Invest. 2004 Dec;114(11):1564-76. doi: 10.1172/JCI18730. J Clin Invest. 2004. PMID: 15578089 Free PMC article.
References
- Libby P, Geng Y, Aikawa M, Schoenbeck U, Mach F, Clinton S, Sukhova G, Lee R: Macrophages and atherosclerotic plaque stability. Curr Opin Lipidol 1996, 7:330-335 - PubMed
- Dollery C, McEwan J, Henney A: Matrix metalloproteinases and cardiovascular disease. Circ Res 1995, 77:863-868 - PubMed
- Lendon CL, Davies MJ, Born GV, Richardson PD: Atherosclerotic plaque caps are locally weakened when macrophages density is increased. Atherosclerosis 1991, 87:87-90 - PubMed
- Nikkari S, O’Brien K, Ferguson M, Hatsukami T, Welgus H, Clowes A: Interstitial collagenase (MMP-1) expression in human carotid atherosclerotics. Circulation 1995, 92:1393-1398 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 HL034636/HL/NHLBI NIH HHS/United States
- P50HL56985/HL/NHLBI NIH HHS/United States
- P50 HL056985/HL/NHLBI NIH HHS/United States
- R37HL34636/HL/NHLBI NIH HHS/United States
- HL 03107/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous