Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer's disease brains - PubMed (original) (raw)
Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer's disease brains
M Q Xia et al. Am J Pathol. 1998 Jul.
Abstract
Chemokines belong to an expanding family of cytokines the primary function of which is recruitment of leukocytes to inflammatory sites. Recent evidence has shown their presence in the central nervous system. Because inflammatory responses have been implicated in the pathogenesis of Alzheimer's disease (AD), we studied the expression of CCR3, CCR5, and their ligands in normal and AD brains by immunohistochemistry. CCR3 and CCR5 are present on microglia of both control and AD brains, with increased expression on some reactive microglia in AD. Immunohistochemistry for MIP-1beta, MIP-1alpha, RANTES, eotaxin, and MCP-3 (ligands for CCR5 and/or CCR3) revealed the presence of MIP-1beta predominantly in a subpopulation of reactive astrocytes, which were more widespread in AD than control brains, and MIP-1alpha predominantly in neurons and weakly in some microglia in both AD and controls. Many of the CCR3+ or CCR5+ reactive microglia and MIP-1beta+ reactive astrocytes were found associated with amyloid deposits. Immunoreactivity for eotaxin, RANTES, and MCP-3 were not detected. Detection of these beta-chemokine receptors on microglia and some of their ligands in reactive astrocytes and neurons as well as microglia suggests a role for this system in glial-glial and glial-neuronal interactions, potentially influencing the progression of AD.
Figures
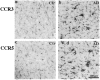
Figure 1.
CCR3 (7B11) and CCR5 (3A9) immunoreactivity in the inferior temporal lobes of a 58-year-old control patient and an 81-year-old AD patient with duration of illness for 12 years (PMIs less than 8 hours). CCR3 (a and b) and CCR5 (c and d) immunoreactivities are clearly seen on microglia of both cases. In the control case, the majority of cells stained are resting microglia, whereas in the AD brain both resting and reactive microglia cells are clearly stained, and some reactive microglia appear in clusters. All images have the same scale of magnification. Scale bar, 200 μm.
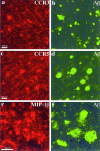
Figure 2.
Confocal images of double immunofluorescent staining. a and b: CCR3 (7B11, Cy3-red) versus Aβ (R1282, bodipy-green); c and d: CCR5 (3A9, Cy3-red) versus Aβ (R1282, bodipy-green); e and f: MIP-1β (1F12, Cy3-red) versus Aβ (R1282, bodipy-green). In a to d, many of the CCR3+ and CCR5+ reactive microglia can be seen associated with Aβ deposits. In e to f, some of the MIP-1β+ reactive astrocytes can also been seen in the vicinity of Aβ deposits. Images a to d are from the same AD case shown in Figure 1 ▶ ; images e to f are from an 81-year-old AD patient with duration of illness for 8 years (PMI, 4 hours). Each of the images is a projection of five Z series images 0.7 μm apart. Scale bars, 50 μm.
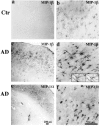
Figure 3.
a to d: MIP-1β (1F12) immunoreactivity in the inferior temporal lobes of a 63-year-old control patient and a 58-year-old AD patient (PMIs less than 8 hours). In the control case, weak MIP-1β staining can be found on a small population of resting and some occasional reactive astrocytes. However, in the AD case, much more widespread and stronger astrocyte staining is observed, and most of the astrocytes appear to be reactive. Insets are high-power images (inverted images) of double staining of MIP-1β (M, Cy3) versus GFAP (G, bodipy), showing a MIP-1β-positive cell is also clearly positive for GFAP. e to f: MIP-1α (11A3) immunoreactivity in the hippocampal formation of an 84-year-old AD patient (PMI, 7 hours). A diffused pattern of neuronal staining can be seen. Both neurons and neuropil were stained. Some neurons showed increased expression of MIP-1α. This pattern of immunoreactivity can be completely blocked by preabsorption with MIP-1α protein. a, c, and e: Low-power images (scale bar, 200 μm); b, d, and f are higher-power images (scale bar, 100 μm).
Similar articles
- Expression of the beta-chemokine receptors CCR2, CCR3 and CCR5 in multiple sclerosis central nervous system tissue.
Simpson J, Rezaie P, Newcombe J, Cuzner ML, Male D, Woodroofe MN. Simpson J, et al. J Neuroimmunol. 2000 Aug 1;108(1-2):192-200. doi: 10.1016/s0165-5728(00)00274-5. J Neuroimmunol. 2000. PMID: 10900353 - Characterization of binding sites for chemokines MCP-1 and MIP-1alpha on human brain microvessels.
Andjelkovic AV, Pachter JS. Andjelkovic AV, et al. J Neurochem. 2000 Nov;75(5):1898-906. doi: 10.1046/j.1471-4159.2000.0751898.x. J Neurochem. 2000. PMID: 11032879 - Macrophage inflammatory protein-1.
Menten P, Wuyts A, Van Damme J. Menten P, et al. Cytokine Growth Factor Rev. 2002 Dec;13(6):455-81. doi: 10.1016/s1359-6101(02)00045-x. Cytokine Growth Factor Rev. 2002. PMID: 12401480 Review. - Neuroinflammation in Alzheimer's disease: chemokines produced by astrocytes and chemokine receptors.
Liu C, Cui G, Zhu M, Kang X, Guo H. Liu C, et al. Int J Clin Exp Pathol. 2014 Dec 1;7(12):8342-55. eCollection 2014. Int J Clin Exp Pathol. 2014. PMID: 25674199 Free PMC article. Review.
Cited by
- Higher Serum DHA and Slower Cognitive Decline in Patients with Alzheimer's Disease: Two-Year Follow-Up.
Chu CS, Hung CF, Ponnusamy VK, Chen KC, Chen NC. Chu CS, et al. Nutrients. 2022 Mar 9;14(6):1159. doi: 10.3390/nu14061159. Nutrients. 2022. PMID: 35334816 Free PMC article. - The key genes, phosphoproteins, processes, and pathways affected by efavirenz-activated CYP46A1 in the amyloid-decreasing paradigm of efavirenz treatment.
Petrov AM, Mast N, Li Y, Pikuleva IA. Petrov AM, et al. FASEB J. 2019 Aug;33(8):8782-8798. doi: 10.1096/fj.201900092R. Epub 2019 May 7. FASEB J. 2019. PMID: 31063705 Free PMC article. - Microglial activation varies in different models of Creutzfeldt-Jakob disease.
Baker CA, Lu ZY, Zaitsev I, Manuelidis L. Baker CA, et al. J Virol. 1999 Jun;73(6):5089-97. doi: 10.1128/JVI.73.6.5089-5097.1999. J Virol. 1999. PMID: 10233972 Free PMC article. - Indomethacin Disrupts the Formation of β-Amyloid Plaques via an α2-Macroglobulin-Activating lrp1-Dependent Mechanism.
Guan PP, Yang LQ, Xu GB, Wang P. Guan PP, et al. Int J Mol Sci. 2021 Jul 30;22(15):8185. doi: 10.3390/ijms22158185. Int J Mol Sci. 2021. PMID: 34360951 Free PMC article. - Differential chemokine alteration in the variants of primary progressive aphasia-a role for neuroinflammation.
Sogorb-Esteve A, Swift IJ, Woollacott IOC, Warren JD, Zetterberg H, Rohrer JD. Sogorb-Esteve A, et al. J Neuroinflammation. 2021 Oct 3;18(1):224. doi: 10.1186/s12974-021-02247-3. J Neuroinflammation. 2021. PMID: 34602080 Free PMC article.
References
- Premack BA, Schall TJ: Chemokine receptors: gateways to inflammation and infection. Nature Med 1996, 2:1174-1178 - PubMed
- Baggiolini M, Dewald B, Moser B: Human chemokines: an update. Annu Rev Immunol 1997, 15:675-705 - PubMed
- Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, Greaves DR, Zlotnik A, Schall TJ: A new class of membrane-bound chemokine with a CX(3)C motif. Nature 1997, 385:640-644 - PubMed
- Fisher SN, Vanguri P, Shin HS, Shin ML: Regulatory mechanisms of MuRantes and CRG-2 chemokine gene induction in central nervous system glial cells by virus. Brain Behav Immun 1995, 9:331-344 - PubMed
- Glabinski AR, Tani M, Tuohy VK, Tuthill RJ, Ransohoff RM: Central nervous system chemokine mRNA accumulation follows initial leukocyte entry at the onset of acute murine experimental autoimmune encephalomyelitis. Brain Behav Immun 1995, 9:315-330 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous